Europe, together with the United States and Asia, is one of the regions in the world where more clinical trials are carried out.
Currently in the US database Clinicaltrials.gov, there are 65,572 registered clinical trials in Europe, 22.13% of which are for cancer treatments (14,509 trials).
What are the reasons that make so many clinical trials in Europe? What advantages do European countries offer?
We explain it to you below.
1. Great experience in clinical trials
Thanks to the fact that so many clinical trials are carried out in Europe, the professionals involved in these studies have extensive experience in clinical research.
This allows the clinical trials carried out to be of high quality.
If you want more information on what are the basic quality aspects that trials must meet, you will find more information in this article: How to carry out high quality clinical trials.
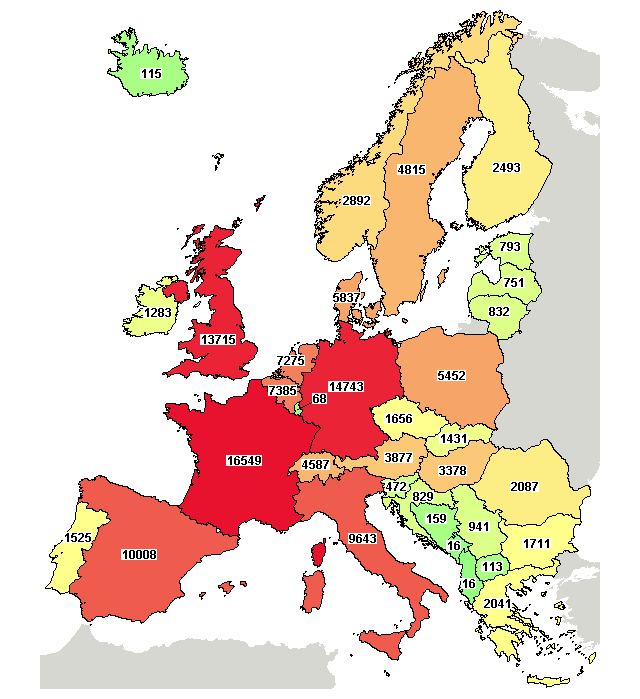
The vast majority of clinical trials are conducted in:
- France(16549)
- Germany (14743)
- United Kingdom (13715)
- Spain (10008)
- Italy (9643)
In the following image you can see the number of clinical trials carried out in each European country (Source: https://ClinicalTrials.gov. Data obtained on 03/07/2019):
![]()
2. Investment of international pharmaceutical companies
It is also important to note that in recent years the interest of international pharmaceutical companies in conducting clinical trials in some European countries (such as Spain) has increased.
In the following video you can see a recent news item highlighting the good conditions of this country to carry out international biomedical R & D projects and how pharmaceutical companies have increased their investment in clinical trials: 15 years ago they invested 299 million euros and in 2017 they reached 662 million.
3. Tests on rare diseases and European Reference Networks (ERN)
One of the advantages of conducting a clinical study in Europe is that there are initiatives and policies that promote research in orphan drugs and rare diseases through economic incentives and other benefits.
Therefore, if your research is related to any of these therapeutic areas, you could benefit from this situation.
In addition, European reference networks (RERs) have been created in complex or rare diseases and disorders that require highly specialized treatment and very specific knowledge and resources.
These networks facilitate the diagnosis and treatment of patients with this type of pathologies, as well as their recruitment for clinical trials.
An example of this is found in ERN EURACAN, the European reference network for adult cancers (solid tumors) that helps patients with rare cancers, that is, tumors that affect less than 6 people out of 100,000 per year.
EURACAN classifies rare cancers in 10 domains following the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD10), to RARECARE and to RARECARENet projects, and these in subdomains.
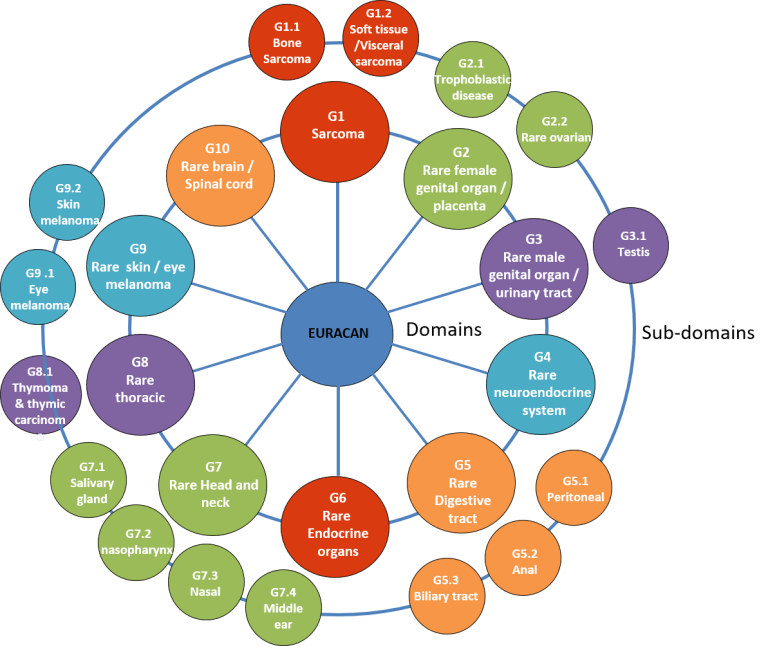
This division facilitates the diagnosis, treatment and investigation of these tumors, since it allows quick access to hospitals and researchers specialized in certain tumor subtypes and speed up clinical trials.
If you want to know more about this topic, in this article you will find information related to clinical trials in rare cancers: 10 Things You Should Know About Rare Cancers and Clinical Trials.
4. The role of cooperative academic research groups
Another factor that can help the promoters to organize and carry out a clinical study in Europe is the presence of several cooperative groups of non-profit academic research in cancer.
In Spain, as a specific example, there are several cooperative cancer research groups and you can learn more about them in this dossier de la Sociedad Española de Oncología Médica (SEOM).
These cooperative groups are organizations formed by oncologists and other professionals specialized in cancer whose objective is to promote research in medical oncology, with a multidisciplinary approach, in a specific tumor type to improve the prevention and treatment of affected patients.
The extensive experience of the specialists participating in these research groups, which more than 20 years ago were created in Spain, facilitates the development of studies and clinical trials with new therapies against cancer is done optimally.
Thanks to them, both the selection of participating hospitals and the recruitment of patients and the start of the trial of your study can be done more quickly.
They are also strongly involved in the dissemination of the knowledge obtained. For example, they collaborate in the creation of guidelines to establish standardized diagnostic and treatment guidelines that can be used in clinical practice.
These groups work closely with international cooperative groups to carry out more extensive studies and achieve better results, thus creating a large network of international knowledge in which European research teams play a key role.
For example, in the World Sarcoma Network, collaborate, among others, the following European groups:
- Grupo Español de Investigación en Sarcomas (GEIS)
- Scandinavian Sarcoma Group (SSG)
- Italian Sarcoma Group (ISG)
- French Sarcoma Group (GSF-GETO)
- German Sarcoma Study Group (GISG)
In the case of European Network of Gynecologic Trials (ENGOT), Among its participating groups we find:
- Grupo Español de Investigación en Cáncer de Ovario (GEICO)
- Multicenter Italian Trials in Ovarian Cancer (MITO)
- Belgium and Luxembourg Gynaecological Oncology Group (BGOG)
- Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe (AGO)
- Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (GINECO)
- Nordic Society for Ginecologic Oncology (NSGO)
5. The legislation of clinical research in Europe
Another of the main advantages of Europe to carry out clinical trials is the legislation that regulates them, since it allows them to be carried out quickly and easily.
Thanks to the regulation about clinical trials with drugs of human use (EU Regulation 536/2014) that entered into force on May 28th 2016, the rules required to conduct clinical trials are the same in the entire European Union.
The fact that this regulation is unified in many countries allows to recruit the desired number of patients with less administrative paperwork. Therefore, this facilitates and speeds up the execution of international clinical trials.
The average time required for the approval of new clinical trials is about 60-90 days. In this article you can find more information about the current situation in Spain: Spain Accelerates Clinical Trial Timelines.
In addition to simplifying the necessary procedures, another of the main objectives of this regulation is to increase the transparency of clinical trials to guarantee the quality of the results obtained.
If you want to know in detail how to carry out a clinical trial in oncology in a European country, we recommend that you download our guide for free: What do you need to carry out a cancer clinical trial in Spain?
6. Bioethics: Standards of Good Clinical Practice (GCP)
Another important point to take into account when choosing where to conduct your clinical trial are the principles of bioethics that research follows, since protecting the safety of participating patients is essential.
The clinical trials conducted in Europe comply with the Good Clinical Practice (GCP) standards of the International Conference on Harmonization (ICH).
It is an international standard that consists of thirteen ethical principles and quality of research whose objective is to protect the rights, safety and well-being of people participating in clinical research, such as clinical trials with drugs and medical devices, in accordance with the principles of the Declaration of Helsinki.
In addition, this regulation also guarantees the reliability and accuracy of the results obtained.
Thanks to the fact that this standard is unified, it facilitates the acceptance by the regulatory authorities of different countries, such as those of the European Union, Japan and the United States, of the clinical data obtained in the investigations.
Unlike what happens in other countries, the legislation that regulates clinical trials in Europe specifies that they must be designed, made and communicated following the rules of BPC, so it is mandatory to comply (not only a recommendation) .
This is another reason for conducting clinical trials in Europe.
If you want to read the complete Good Clinical Practice guide, you can access it from here
7. The quality of health systems and research in European countries
It is also necessary to assess the quality of the health systems of the countries in which the clinical trial is to be carried out, since the reliability of the results obtained depends to a large extent on this.
The majority of European countries have health systems of excellent quality.
You can check this on the following map, which shows the Healthcare Access and Quality (HAQ, for its acronym in English) of each country in 2016.
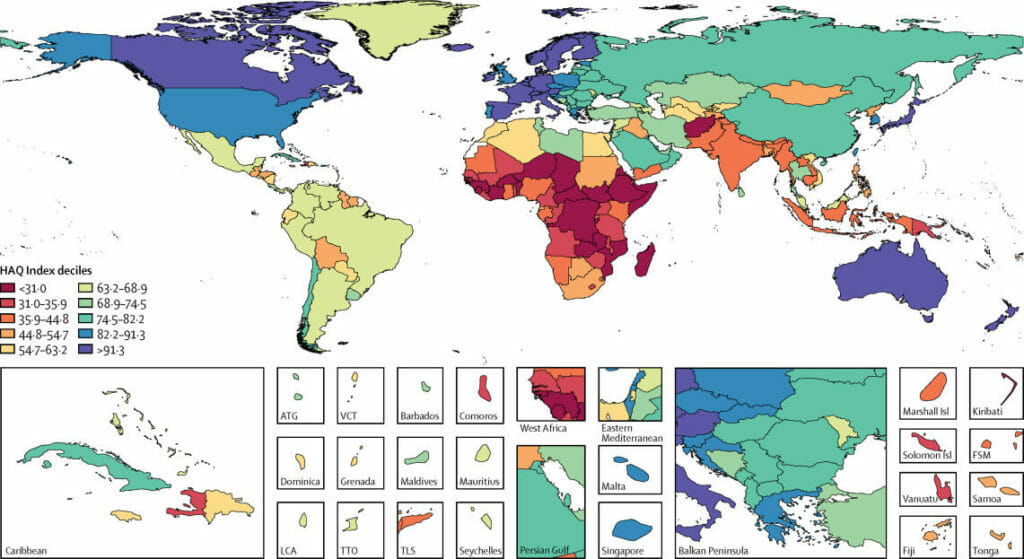
The study was published in The Lancet and, if you want detailed information, such as the HAQ index of each country, you can access the full article from this link: Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016.
Thanks to the good organization of health systems and the extensive research experience available to professionals in most European countries, it is possible to carry out high quality clinical trials and obtain totally reliable results.
This feature also facilitates the recruitment of patients for clinical studies.
Conclusion: Why is Europe a good place to conduct clinical trials?
As we have seen in this article, Europe is a very interesting territory to consider in order to develop a clinical trial. The main reasons are:
- The professionals involved in clinical research have extensive experience.0
- International pharmaceutical companies increasingly invest more money in clinical trials in these countries thanks to their good conditions.
- Many resources are being devoted to the study of rare diseases, such as rare cancers, and thanks to European referral networks it is easier to recruit patients.
- There are cooperative academic research groups in cancer that promote research in medical oncology.
- Agility and transparency in the bureaucratic procedures necessary to carry out your research thanks to the legislation that regulates them.
- Good Clinical Practice standards ensure that clinical trials protect the safety, well-being and rights of participants and that results are reliable.
- Europe has quality health systems, which helps to obtain reliable results.
For all these reasons we believe that you should consider Europe to carry out your clinical trial.
If you want more information about the advantages of conducting your clinical oncology trial in Spain in particular, we recommend that you download our free guide at the following link: Nine reasons to conduct an oncology trial in Spain.

At Sofpromed we can help you carry out your clinical trial in Europe. If you have any questions about how to perform your clinical trial, you can write us a comment here or contact us at info@sofpromed.com