Rare tumors represent a unique challenge in oncology, accounting for a small proportion of all cancer diagnoses but encompassing a wide variety of cancer types.
These tumors are often difficult to study due to their low prevalence, resulting in limited understanding of their biology, progression, and optimal treatment strategies.
Patients with rare tumors frequently face fewer treatment options and a lack of standardized care pathways, underscoring the urgent need for clinical trials tailored to these cancers.
Conducting research in this area is crucial to uncovering new therapies, improving outcomes, and addressing the unmet medical needs of this underserved population.
Clinical trials for rare tumors require innovative approaches, including international collaboration, adaptive trial designs, and biomarker-driven studies, to overcome challenges such as small patient populations and geographical dispersion.
By advancing research and therapeutic development, clinical trials play a vital role in transforming the care of rare tumor patients and providing hope for improved survival and quality of life.
Rare cancers represent nearly 20% of all cancer cases. Many of them do not have effective treatments. Are there clinical trials for rare tumors?
If you are looking for comprehensive and reliable information about rare cancers, and particularly on clinical trials conducted for these tumor types, you are in the right place.
We hope this material will contribute to increase awareness on rare tumors, helping patients, researchers, clinical trial sponsors, and society in general.
1. What Is a Rare Cancer?
According to the National Cancer Institute of the United States, a rare cancer is a tumor that affects fewer than 15 out of 100,000 people per year.
Rare cancers belong to the group of rare diseases. Rare diseases are normally defined as those with a prevalence of less than 50 out of 100,000.
Experts say that a cancer is considered “rare” if less than 6 in 100,000 people are diagnosed each year. This implies, for example, that 1 out of 5 persons with cancer in Europe has a rare tumor.
Even when considering cancers with an incidence near 5/100,000/year, rare tumors still represent about 22% of all cases of malignant neoplasms.
2. How Do Rare Tumors Affect People in the United States?
Around 20% of patients with cancer in the United States are diagnosed with a rare cancer.
The article “The burden of rare cancers in the United States” indicates that infrequent tumors constitute a significant number of cancers in Hispanic (24%), Asian/Pacific Islander (22%), non‐Hispanic blacks (20%) and non‐Hispanic whites (19%).
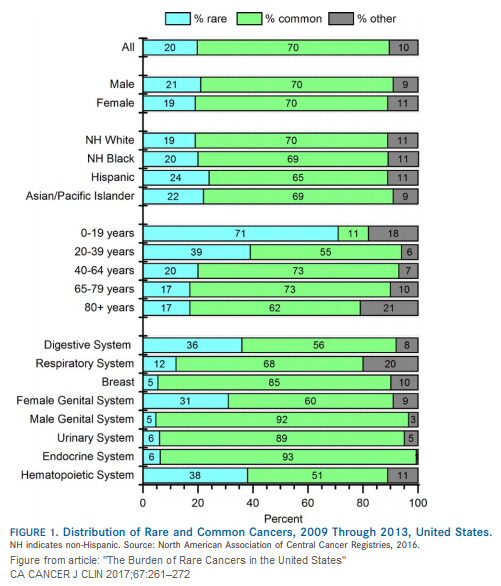
In addition, more than 71% of cancers observed in children and adolescents are rare cancers compared with less than 20% of cancers diagnosed in patients aged 65 years and older.
3. How Is the Situation of Rare Cancers in the European Union?
Looking at the excellent work of the RARECARE project, we see that around 4.3 million people in the European Union (EU) have a rare cancer. More than 500,000 new cases are registered every year.
Taken together, rare tumors represent about 22% of all cancer cases annually diagnosed in the EU.
These numbers include rare adult solid tumors (13%), rare haematological cancers (8%), and childhood malignancies (1%).
4. How Do Rare Tumors Affect People in the Rest of the World?
In Australia, in 2017, nearly 52,000 people were diagnosed with a rare cancer and 25,000 died from them, according to Cancer in Australia estimates.
In Japan, the sum of all rare cancers accounts for about 2 out of 10 new cancers diagnosed in the country.
A major problem with rare tumors is that their real impact on population has not been studied in depth in many countries worldwide.
Most rare cancer registries and clinical trials have been developed in Europe and North America only.
Therefore, rare cancer data in other regions, such as Latin America or Africa, is quite scarce.
We recommend the interesting article “Rare tumor research in emerging countries” to better understand the reality of these cancer types in developing regions.
5. How Many Rare Cancer Types Are There?
In general, there are more than 200 types of cancer (considering common and uncommon ones), of which nearly 190 are considered rare.
Rare Cancers Europe provides a very useful list of rare cancer families here.
The RARECARE project has performed outstanding research on rare cancer types, and also gives access to this detailed spreadsheet including incidence rates.
Note that rare cancers are those with incidence less than 6/100,000.
6. What Are the Challenges in the Treatment and Research of Rare Tumors?
The document “Improving Rare Cancer Care in Europe Recommendations on Stakeholder Actions and Public Policies” issued by the European Society for Medical Oncology (ESMO) describes in detail the management and research challenges linked to rare cancers.
Do not miss this interesting video in which Prof. Jean-Yves Blay discusses some of the difficulties of rare tumor care:
The main problems related to rare cancer management are:
- Patient access to treatments may change a lot across and within countries, which leads to unfortunate social inequalities
- Information about rare tumors, their therapies and where to obtain appropriate care is, frequently, not available to patients
- Not so good treatment outcomes are common for rare cancers due to a lack of medical expertise
- Poor referral rates from general practitioners to specialized reference centers worsen clinical results
- Pathologic misdiagnosis has a negative impact on subsequent patient treatment decisions
- Outcomes for rare cancers could be improved through the establishment of reference networks or centres of expertise
- Few reference networks or centres of expertise exist
- Health costs can be much higher for patients with rare cancers
- Referrals for second opinions are not a common practice
- Many patients must travel long distances to access appropriate care
- Exchange of experience, information and data on rare cancers is infrequent
- Registries and biological sample banks for rare cancers are scarce
- Clinical studies are more difficult to conduct in rare cancers due to the low number of patients
- The selection of experimental treatments in clinical trials for rare tumors is often based on less evidence (possibly weaker rationales)
- There has been a lack of interest by pharmaceutical companies in developing new therapies for rare tumors since the market is smaller (less revenues)
We highly recommend the following video, “Rare Cancers: Charting a Faster Route to Treatment”, which includes interesting reflections on what can be done to have more effective treatments for rare tumors:
7. Rare Tumors Have Worse Survival
According to a population-based study on survival from rare cancer in adults, there is evidence that five-year survival rates are worse for rare cancers (47%) compared to common cancers (65%).
Only a few treatments have been developed for rare tumors so that while survival has improved a lot for common cancers, there has not been much progress in survival for patients living with rare malignancies.
For example, the 5-year survival rates for soft tissue sarcomas (a type of rare cancer) have not changed much for many years. According to the American Cancer Society, the 5-year survival rates are:
- 81% for localized sarcomas
- 58% for regional stage sarcomas
- 16% for metastatic sarcomas
8. Are There Clinical Trials Available for Rare Tumors?
Yes. There is an increasing number of clinical trials available in rare cancers.
The American and European clinical trial databases can be used to find open trials by tumor type (insert the tumor name and click on “Search”):
9. The Important Role of Academic Cancer Research Groups
Academic research groups are non-profit scientific entities formed by medical oncologists and other clinical and translational specialists who come together in order to promote research and carry out clinical trials in different tumors.
Spain is a good example of a European country with several cooperative research groups dedicated to cancer research:
- GECP – Spanish Research Group in Lung Cancer
- GEICAM – Spanish Research Group in Breast Cancer
- GEICO – Spanish Research Group in Ovarian Cancer
- GEINO – Spanish Research Group in Neuro-oncology
- GEIS – Spanish Sarcoma Research Group
- GEM – Spanish Melanoma Multidisciplinary Group
- GEMCAD – Spanish Multidisciplinary Group in Digestive Cancer
- GETHI – Spanish Group of Orphan and Infrequent Tumors
- GETICA – Spanish Group of Immuno-Biological Therapies in Cancer
- GETNE – Spanish Group of Neuroendocrine and Endocrine Tumors
- GOTEL – Oncological Group for the Treatment of Lymphoid Diseases
- ICAPEM – Association for Lung Cancer Research in Women
- SOGUG – Spanish Group of Genitourinary Oncology
- SOLTI – SOLTI Group for Research in Breast Cancer
- TTCC – Spanish Group for the Treatment of Head and Neck Tumors
- TTD – Digestive Tumors Treatment Group
Some of these groups are completely dedicated to rare tumors, while others may investigate rare subtypes along with other more common histologies.
10. How to Conduct Effective Clinical Trials in Rare Cancers
Conducting clinical trials in rare cancers implies important challenges, and that is why there are fewer clinical trials for rare tumors than for more common ones.
First, it is difficult to run trials for rare cancers because it can take a long time to recruit the number of patients needed.
Enrolling sufficient patients is essential to the success of a trial. The results will not be solid enough if the study only involves few patients.
The fact is that new therapies are urgently needed for patients with rare tumors, in particular for those with metastasis.
In some rare cancers, complete responses to chemotherapy are seldom seen and survival is short.
Current chemotherapies are simply not effective while just a few drugs have been approved (even in the last decades).
Then, what are the keys to conduct an effective clinical trial in rare tumors?
- Restricted patient selection. Inclusion criteria must be limited to the specific histologic subtype(s) to be studied. Specific patient selection (tumor diagnosis homogeneity) is essential for the emergence of new successful compounds in rare tumors.
- Accurate diagnosis. Clinical trials must guarantee correct diagnosis by expert pathologists. If you are not able to ensure the right diagnosis of rare tumors, then the study will not generate trustworthy results.
- International collaboration. Trials in rare cancers require a multinational effort by cooperative networks and reference centers with high volume of patients since the target population can be very low.
- Multidisciplinary approach. Clinical studies in rare tumors must be built on a multidisciplinary strategy. These studies must count on the collaboration of different professionals, such as oncologists, pathologists, surgeons, radiologists, and molecular biologists, since rare tumor management is complex.
- Biomarkers. Clinical trials should be designed integrating translational substudies focused on the identification of biomarkers of treatment response. This is fundamental to develop more targeted therapies.
- Work with national research groups. Clinical trial sponsors should consider collaborating with national research groups already specialized in recruiting patients with rare tumors in each country. Why selecting hospitals from zero, when well organized hospital networks have already been recruiting patients for years? Thus sponsors can benefit from fast recruitment, local know-how, and expert medical guidance.
- Make the results available. Current clinical trials should facilitate future access to rare tumor data and biological samples, to further characterize these cancers. The research done today will help tomorrow’s researchers.
Let’s get together in the battle against rare cancers. Hopefully, soon we will have new treatment options for those patients not having many today.
For further questions regarding rare cancers and clinical trials, please contact us at info@sofpromed.com











