Vlad Bogin is the Chief Executive Officer of Cromos Pharma, a full-service clinical research organization (CRO) with headquarters in the United States and strong regional presence in Central/Eastern Europe.
Cromos Pharma offers clinical trial management services for the biotech and pharma industries, with local footprint in the United States, Australia, Belarus, Bulgaria, Czech Republic, Estonia, Georgia, Hungary, Israel, Latvia, Lithuania, Moldova, Poland, Romania, Russia, Slovakia, Ukraine, and Ireland.
In this interview with Vlad we are going to review the current landscape of clinical trials in Eastern Europe, providing useful recommendations to sponsors considering this part of the world to carry out their clinical studies.
Patricio Ledesma (PL): Dear Vlad, thank you for participating in this interesting conversation about conducting clinical trials in Eastern Europe.
Vlad Bogin (VB): Thank you Patricio! It is my pleasure to talk to you. I am partial to this topic and I hope your readers will enjoy our discussion.
PL: Firstly, could you tell us about your academic background and expertise? How did you get started in clinical research?
VB: I first got acquainted with clinical research as a chief medical resident at the University of Rochester. I was asked to help on a clinical trial as a sub-PI. It was an ICU study and we were putting drug-eluting central venous catheters that were supposed to reduce the incidence of blood infections. I found this experience to be fascinating and when I became an attending I almost immediately started consulting for biotech and pharma. I subsequently joined Boehringer Ingelheim as a Medical Director in Cardiovascular department where I oversaw late stage clinical trials with a novel blood thinner. Patient recruitment seemed to always be a challenge and I soon realized that there existed a tremendous unmet need in taking large clinical programs ex-US. I started Cromos after leaving BI and have been growing the company for the last 16 years. Given that I am originally from Moscow this is where I went first. The company started with a one-room office and 3 employees and organically grew to a mid-sized organization with around 150 employees.
PL: What is Eastern Europe? Which countries constitute this region?
VB: Great question as definitions do vary. The United Nations classifies “Eastern Europe” as including the following countries: Belarus, Bulgaria, Czech Republic, Hungary, Moldova, Poland, Romania, Russia, Slovakia and Ukraine.
Central Europe is defined as Austria, Belgium, the Czech Republic, Denmark, France, Germany, Hungary, Ireland, Liechtenstein, Luxembourg, the Netherlands, Poland, Slovakia, Switzerland and the United Kingdom.
Do you see the overlap?
And then there is the former “Eastern Bloc” that includes Poland, Czechoslovakia, Hungary, Romania, Bulgaria, Albania, plus/minus Yugoslavia.
Many still think of Eastern Europe as encompassing all the former Eastern Bloc countries but, as you can see, this isn’t the case. For the purposes of this discussion, let’s define our region to the following countries of Central and Eastern Europe: Belarus, Bulgaria, Czech Republic, Hungary, Moldova, Poland, Romania, Russia, Slovakia and Ukraine. And let’s call it CEE going forward.
PL: Could you explain the main advantages of conducting clinical studies in these territories?
VB: Definitions aside, CEE is the home to about half a billion of potential study subjects. Subjects that are quite eager to participate in clinical research. In a nutshell, this is the largest attraction of the region.
There is one significant difference in how healthcare is delivered in the former Eastern Bloc. While the local approaches have changed somewhat since the fall of Communism, the general principles of “Semashko model” remain. It consists of rather generic primary screening at the polyclinic level with subsequent referrals to specialty and subspecialty clinics and hospitals that specialize in a single field of medicine. Thus, it is not uncommon to find a thousand bed oncology or cardiology hospitals in the major cities across the region.
As a CRO you cannot find a more fruitful soil for clinical research. Just imagine – you instantly get access to literally hundreds of patients with a disease pathology that you are interested in. This is the reason why in international trials CEE consistently recruits 2-3 times more patients that Western Europe and the US.
The data quality that comes out of CEE is very high. And don’t take my word for it. If you tally the official results of FDA and EMA inspections, adjust it for cumulative incidence, you will see that this region generates far fewer major findings than the US and the Western Europe. I am often asked to explain this phenomenon. I believe that the high quality is the product of high investigator and patient motivation. It is a fact that physicians’ salaries in Eastern Europe are modest and hence the investigator grants are much more impactful to their financial bottom line. This translates into great attention to detail when working with trial data. In fact, unlike the US, where trial nurses are responsible for data entry, many investigators in Eastern Europe choose to fill out eCRFs themselves.
The patients also view the participation in clinical trials very favorably. In some instances, being in the trial offers better quality of care, removes out of pocket expenses and allows access to the most advanced and experimental treatment protocols. As a result, the compliance is high, and the dropout rates are low.
The costs of conducting clinical trials is also much lower than in WE and the US. Based on our internal analysis, the investigator grants are 40-50% lower than in the US and CRO costs are at least 40% lower, unless you select one of the top global providers.
To summarize – Central/Eastern Europe boasts a large patient population, tremendous recruitment, motivated investigators, compliant patients and high data quality. And all of this comes with a significant discount. These are the variables that are hard not to like.
PL: All of this sounds quite encouraging but there is still some apprehension about conducting clinical trials in this part of the world. Can you talk about the common ones?
VB: Absolutely. While over the last 20 years there has been a tremendous influx of studies into Central/Eastern Europe, there are still some stigmas that exist.
One of the most common questions that we are asked by the sponsors is about clinical trial standards and applicability of ICH-GCP. They get relieved when we tell them that in all local jurisdictions the national clinical trial standards are a direct translation of ICH-GCP.
Their next question is often about whether the clinical data generated in CEE is accepted by the FDA and the EMA. The answer is a resounding “yes”. At least 70% of large scale international pivotal trials engage CEE sites and often have a bulk of patients recruited there.
And lastly, there are concerns about the technical capabilities of the medical facilities. These concerns are valid and, depending on the complexity of the trial, we have to perform a diligent feasibility analysis to make sure that the sites that we present to the sponsor have adequate equipment and adhere to the standards of care that would satisfy the protocol. For instance, not every site in CEE will have a PET/MRI scanner. However, if the site is well known to us, has a good track record, we find ways of organizing the necessary imaging at adjacent sites that have such capabilities. This is where local knowledge and experience become critical.
PL: What are the CEE countries with the highest number of clinical trials?
VB: Based on the data from clinicaltrials.gov and clinicaltrialsregister.eu the most prolific countries in clinical research are Poland, Hungary and Russia. I am happy to say that we are successfully operating in all of them.
PL: What are the main differences of performing clinical trials in Eastern Europe versus the United States?
VB: Good question and having operations in both locales I hope that I have the right expertise to answer it. Clearly, there are certain cultural, organizational and logistical aspects that are endemic to CEE and to the US. As I have mentioned, the medical facilities in CEE are often super-specialized and deal with one medical specialty. For instance, in Moscow we work with the Bakulev Cardiovascular Center, a vast facility that performs more open-heart surgeries than any center in Western Europe. Thus, working in CEE can spoil you – unlike the US where you get a few patients from many centers, in CEE you frequently get many patients from a few very large centers.
On the other hand, there is clearly more experience with cutting edge technologies and treatments in the US and the process of getting the investigators acquainted with the protocol and the study procedures is less laborious.
There is one caveat that I have omitted when speaking about pros and cons of CEE and that is availability of concomitant and comparator medications for the trial and its reimbursement. There are instances where certain cutting edge and expensive drugs aren’t fully adopted and are thus not covered by the national insurers. In such situations the sponsor must pick up the cost of these drugs. This increases the cost of the study, but it is always offset by lower CRO costs, lower investigator grants, and, indirectly, by faster recruitment and trial execution. At times there is a silver lining to these deficiencies. There are situations where the sponsor is unable to run a study against an older standard of care because the uptake of the new treatment is so robust in the US. This is where moving to CEE becomes critical.
PL: Interesting. Are there any other circumstances where you would recommend conducting trials in one region versus the other?
VB: There are certain fields of medicine that aren’t as developed in CEE as they are in the US. One example is solid organ and bone marrow transplantation. The numbers just don’t match and coming to CEE for fast recruitment is clearly not feasible. We are always transparent with our clients and our recommendations are always based on thorough feasibility analysis of each trial and each venue. If we feel that the trial is better suited for the US, we are the first to suggest that to the sponsor. Nevertheless, most protocols that we see are equally suited for the US and the CEE and we frequently recommend both locations that saves our clients time and money.
PL: Are Eastern European countries particularly advantageous to conduct clinical studies in specific diseases? What about rare diseases?
VB: As I stated earlier, CEE is a strong choice for most therapeutic indications. Rare diseases aren’t an exception and frequently the sponsor must go ex-US or ex-WE to complete the recruitment. We recommend that they do it sooner rather than later. Our CEE capabilities are frequently used for “study rescue” and you never want to be in the position where your trial needs to be rescued. Thus, when the sponsor comes to us with a protocol or even a synopsis of a proposed trial, we often suggest at least a feasibility analysis of CEE locations. After such consultations it is quite common for the sponsors to change their initial approach and to include CEE from the outset. We are yet to see a single sponsor who regretted such a decision.
PL: As mentioned, Eastern Europe is composed of several nations. What are your key service capabilities? How does Cromos Pharma help biotech companies plan clinical studies in this part of the globe?
VB: Cromos Pharma is a full-service client-centric mid-sized CRO. Our services include the usual spectrum, from medical writing, to regulatory support, to data management, project management and clinical monitoring. When you come to us you get a very comprehensive assessment of your clinical program. We love medicine and love to get involved before the protocol is finalized and before the IND is cleared. Often, we can weigh in on the design so that the study can run efficiently across multiple geographies. We are laser focused on feasibility and have a very strong team that performs a detailed analysis, starting with getting KOLs’ opinions, sending questionnaires to the prospective investigators and sites, and evaluating competing programs. At the end we provide our clients with a comprehensive feasibility assessment report that includes our suggestions and ranks the potential countries and sites based on multiple criteria.
PL: How is Cromos Pharma different from other CROs? What is your particular added value?
VB: We are the only CRO with a unique risk-sharing program called “No Patients – No Payment” that basically means that you don’t pay unless we enroll. Obviously, we go through a very detailed feasibility assessment and also make sure that your expectations match our capabilities; if we arrive at consensus we put this in writing, and it becomes part of our contract with the sponsor. This is the most honest way of showing the client that we put our money where our mouth is. Please note that we can only do this if we are the ones who performed the feasibility and if the sponsor agrees with our country/site selections.
We do care about our reputation tremendously. And that means that we are completely open and honest with our sponsor from day one. We are not making cheap proposals to win a project just to flood the sponsor with change orders later. This is probably the reason why our return business rate is consistently at over 80%.
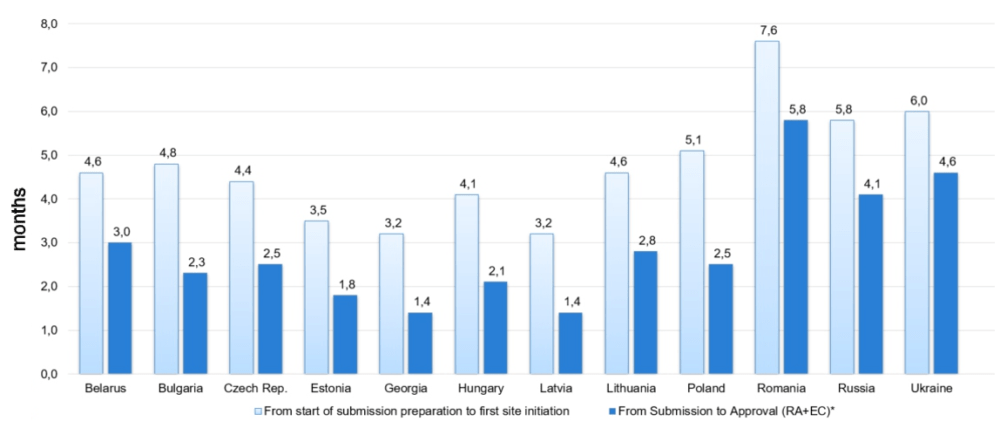
PL: How long does it take to initiate a clinical trial in Eastern Europe? Could you describe the average study start-up timelines in the countries in which you provide service?
VB: Not only I can describe it, I can show it to you in a graph. These numbers are real and are based on our experience. Here you can see the duration of the approval process and time to first site initiation. Overall, these timelines are very competitive with WE and are much more palatable that many other regions that we compete with.
PL: Let’s consider a biotech company from the United States or Asia planning its first large phase 3 multinational clinical trial including Eastern Europe countries. What would be your practical advice when planning such study?
VB: Again, if we can opine on the protocol before it is finalized, we may positively affect both the future recruitment and the regulatory clearance.
I recommend starting the dialogue with the KOLs, the investigators and the sites as early as possible. Their feedback could be extremely valuable.
Whichever CRO the sponsor selects, they should provide a comprehensive startup plan that should include their approach to the regulatory submissions, ethic committee approvals, site and investigator contracts, drug and depot logistics, patient insurance, etc. Things do vary from country to country; for instance, in Poland contract negotiations can be lengthy and should be started in parallel with the regulatory dossier submission. Russia is very particular when it comes to review of preclinical studies, and so on. Having an experienced CRO with local roots and strong working relationship with both the investigators and the regulatory authorities can make a dramatic difference.
PL: Is Eastern Europe a strategic region to carry out cancer clinical trials? In which tumor types do you have expertise? Can Cromos Pharma facilitate the selection of clinical sites and investigators in Eastern Europe for cancer studies?
VB: As I have explained Eastern Europe is more than suitable for all types of trials, including Oncology. While Cromos is not a niche CRO, given that oncology studies have been dominating the clinical trial arena, oncology is our top indication and we have conducted over a hundred of late stage clinical programs in both solid and hematologic cancers. The only trials that we would approach with caution and would perform at least a cursory capability assessment before we sit down with the sponsor are the trials that include bone marrow transplantation, graft vs. host disease and very sophisticated treatments that require complex biosample processing, such as CAR-T trials.
Other than that, we are unbeatable in terms of recruitment and even during COVID we have been able to recruit the record numbers of patients into some very challenging programs.
PL: Is there any additional piece of advice you would like to transmit to biotech CEOs considering CEE as a destination for their drug development programs?
VB: Not all trials should include CEE, but at least 90% should. Neither blind optimism nor the outright dismissal are the right attitudes towards this very attractive region. Carefully select the right service partner, make sure that you are receiving the correct information and do your own SWAT analysis. And 9 times out of 10 the benefits would outweigh the risks.
PL: Vlad. I am very thankful for this instructive conversation. Thank you so much for your valuable insights.
VB: It was my pleasure, I hope you and your readers will stay safe and well. Please stay hydrated and take vitamin D!
About Cromos Pharma
Cromos Pharma combines global expertise with in-depth experience and knowledge in Central and Eastern Europe to offer exceptional patient recruitment. In our 16 years history, their team has met or reduced enrollment timelines in 95% of trials conducted.
For further information about conducting clinical trials in Eastern Europe, please contact us at: info@sofpromed.com