Pharmaceutical formulation is an important challenge faced by biotech companies developing new drugs to be tested in first-in-human (FIH) clinical trials.
Formulation development is a fundamental factor not only in the early stages of drug development, but also in the future marketing success of a promising investigational medicinal product.
This article provides a practical introduction to drug formulation development aspects —in the context of early phase clinical trials— and seeks to help biotechnology companies in this key process within the whole drug development journey.
What is drug formulation?
Drug formulation —also known as pharmaceutical formulation— is the process through which a variety of substances are combined with the drug’s active pharmaceutical ingredient (API) to finally produce a drug product that can be successfully given to patients.
Why is drug formulation development important?
Formulation development is a process of utmost importance as it involves developing an adequate preparation and form of a drug, which is both stable and acceptable to the patient.
In 2018, Informa Engage —on behalf of Informa Pharma Intelligence, Rentschler Biopharma SE and Leukocare AG— conducted a survey to evaluate the status quo, importance, and future of formulation in the drug product development process.
The survey included qualified responses from participants active in the biopharmaceutical industry.
A variety of professionals from the pharma and biotech industries was represented in the survey, with clinical development and drug product development being the most common profiles.
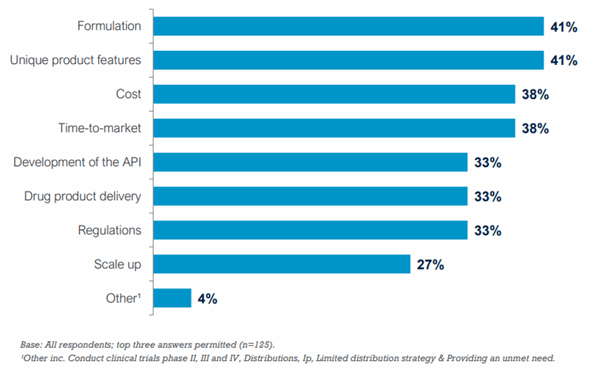
Formulation and the unique features of new compounds were the top two most important considerations in drug product development.
Most Important Considerations in Drug Product Development
What aspects should be considered at the beginning of the formulation development process?
A critical part of the drug formulation planning stage is deciding what physical form the medicinal product should have.
Without doubt, simplicity and ease of administration must be top priorities when choosing the form of a medicine, in order to ensure successful usage by patients in early phase clinical trials and in the clinical setting at a later stage.
Biotech companies must consider what drug form will be the least disruptive, so patients must be kept in mind since the very beginning of the drug development process.
What specific drug dosage forms are available in the market?
In the area of solid drugs, biotech companies can consider options such as capsules and coated tablets, among others.
When it comes to liquids, some examples are oral liquids or injectable vials.
In the non-oral drug types, there are topical drugs, such as creams, gels, eye drops, nasal drops, inhalers, and others.
You may want to see this presentation by S. Bohlooli for further information about dosage forms.
What are drug preformulation studies?
According to Gita Chaurasia:
“Preformulation is a group of studies that focus on the physico-chemical properties of a new drug candidate that could affect the drug performance and the development of a dosage form.
This could provide important information for formulation design or support the need for molecular modification.
Every drug has intrinsic chemical and physical properties which have been considered before development of pharmaceutical formulation.
This property provides the framework for drugs combination with pharmaceutical ingredients in the fabrication of a dosage form.
The objective of a preformulation study is to develop the elegant, stable, effective and safe dosage form by establishing kinetic rate profile, compatibility with the other ingredients and establishing physico-chemical parameters of new drug substances.
Among these properties, drug solubility, partition coefficient, dissolution rate, polymorphic forms and stability play an important role in a preformulation study [1].”
What are some examples of drug formulation challenges during early phase development?
Stability and bioavailability are common challenges faced in early phase drug development programs.
Drug product stability may be defined as the capability of a particular formulation to remain within its physical, chemical, microbiological, therapeutic, and toxicological specifications.
Bioavailability is the fraction of an administered dose of a drug that reaches the systemic circulation as an intact drug and the rate at which this occurs.
A compound might have deficient stability in a solution and will need to be combined with another substance before giving the drug to the patient.
Inadequate solubility can also have an impact on bioavailability.
More than 40% of new chemical entities [2] and a high percentage of APIs in development are poorly soluble.
Optimization of drug solubility and bioavailability of drugs stands as one of the major difficulties faced by the biopharmaceutical industry today.
Several new compounds in clinical development are facing challenges in late phase clinical trials due to suboptimal bioavailability.
Why is it important to keep later stage clinical trials in mind when starting a drug development program?
When creating a drug formulation it is very important that late stage development is kept in mind.
Relevant stability and bioavailability data can guide the development of formulations for later phase studies, and optimize manufacturing when the compound reaches the clinic.
Many first-in-human clinical trials use liquid formulations of drug candidates since they are faster and cheaper to develop.
Moreover, liquid formulations are often seen as a way to maximize oral bioavailability.
However, the feasibility to develop a future solid product should be carefully considered during the development of formulations for early phase clinical studies.
Solubility and bioavailability may be difficult to reproduce in more complex dosage forms used in late phase clinical trials and in the actual clinical setting.
What are the chances of drug development failure or delay due to formulation problems?
Biotech companies must know that deficiencies in formulations can lead to failures and significant delays in the drug development process.
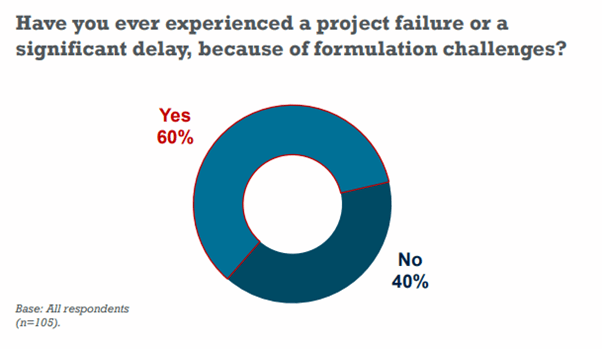
In the Informa Engage survey mentioned above, 60% of pharma and biotech professionals answered “Yes” to the question of having experienced a project failure or a significant delay, due to formulation challenges.
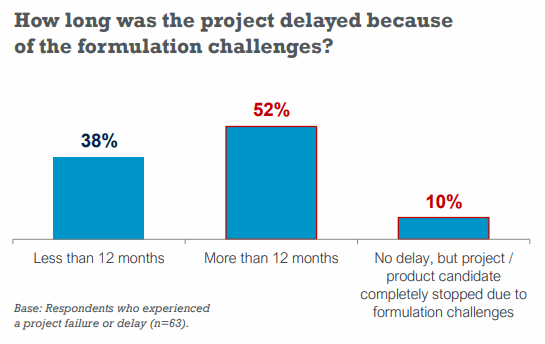
Up to 52% of respondents said they had a delay of more than 12 months because of formulation issues.
This data shows the major impact that formulation development can have on a drug development program.
What are drug deformulation studies?
A deformulation study —also known as “reverse chemical engineering”— is the process of assessing the formulation of a drug product to separate and specify the identity and precise amount of its components (including active pharmaceutical ingredients —APIs— and excipients).
Deformulation is performed to replicate existing products, obtain detailed information about drugs, identify discrepancies in batch-to-batch products, and fix product deficiencies.
Deformulation work can be used to analyze and reorganize a wide range of drug products, from solid oral forms to injectables, among many others.
Why should biotech companies outsource drug formulation development?
Biotech companies should outsource formulation development work for two main reasons: development acceleration and access to specialized knowledge.
Formulation development activities take time and biotech companies may not have the staff resources to face such type of work.
In some companies, formulation and analytical departments may get overloaded due to the increasing number of projects.
Moreover, timeline prediction in development projects requires skill in assessing laboratory procedures that may be hard to foresee.
Outsourcing these tasks to experts and expecting a contract development and manufacturing organization (CDMO) to meet the development deadlines may be more efficient and trustworthy.
CDMOs normally have the flexibility to assign staff quickly to new projects even with pressing deadlines.
Another evident reason to externalize formulation development is to count on the specialized expertise of CDMO professionals.
For example, certain CDMOs may have extensive experience and deep knowledge in assessing and optimizing the solubility and stability of specific compounds, and they may be very familiar with common technical issues, since they have a long list of similar projects in their record.
Selecting an already available expert team with proven expertise, clearly competent in your project type, simply makes sense, saves time and money, and ensures technical proficiency for your development program.
Conclusion
Formulation development is essential for the success of a drug.
Formulation-related challenges include stability and bioavailability issues, among others.
When defining a drug formulation it is very important that late stage development is kept in mind from a very early phase.
Biotech companies must know that deficiencies in formulations can lead to costly failures and extended delays in the overall drug development process.
Outsourcing formulation work provides drug development acceleration and access to specialized knowledge, which are very important advantages biotech companies should benefit from.
Finally, below you can find a very interesting presentation entitled “Enabling Formulation Technologies for Improving Oral Absorption of Poorly Water Soluble Drugs” by Dr. Ping Gao.
Please contact us at info@sofpromed.com if you have any questions or comments, or if you need drug formulation development services. You can see a description of our drug formulation services here.
References