Post-approval studies —also known as postmarketing or post-authorization studies— are important clinical studies conducted by biotech and pharma companies once a drug has obtained a marketing authorization.
In this article we will discuss post-approval studies in detail, providing valuable guidelines to conduct these types of studies in Europe (taking Spain as an example).
What is a post-approval (PAS) study?
A post-approval study is a clinical study performed by biotechnology and pharmaceutical companies seeking to generate additional safety and efficacy data of a drug, after it has been approved for commercialization by regulatory authorities.
What are other names for post-approval studies?
Post-approval studies with medicines are also known as postmarketing or post-authorization studies, emphasizing the fact that they are done after a drug has received a marketing authorization.
In addition, post-approval studies may be referred to as “phase IV clinical trials”, as they are executed after phase III clinical trials.
Are post-approval studies the same as “observational studies”?
An observational study in clinical research is a non-interventional study that only “observes” what is happening in approved treatments, therefore not implying experimental interventions.
Since post-approval studies are performed with approved drugs in authorized diseases and conditions of use, they can be considered as “observational studies”.
However, although post-approval studies are observational studies, not all observational studies are “post-approval”.
For example, you can have an observational study not even including the use of a drug (e.g. observing the evolution of a specific disease during a period of time), and therefore this kind of study is not “post-approval”.
Why are post-approval studies needed?
Post-approval studies are needed to generate additional information about a marketed drug, usually data related to safety and efficacy aspects.
Before a medicine is authorized to be sold, it is tested in phase 1, 2, and 3 clinical trials.
However, the three phases in drug development may not be enough to collect long term safety data.
Therefore, it is common to use post-approval studies to evaluate potential drug-related adverse events or toxicities that may take place in the long run.
What is the main difference between a post-approval study and a clinical trial?
The main difference between a post-approval study and a clinical trial is that a post-approval study has a non-interventional nature, in contrast with the interventional nature of clinical trials.
In other words, postmarketing studies do not include experimental interventions, just the observation of a therapy that has already been authorized.
On the other hand, a clinical trial includes an experimental treatment that has not been yet approved.
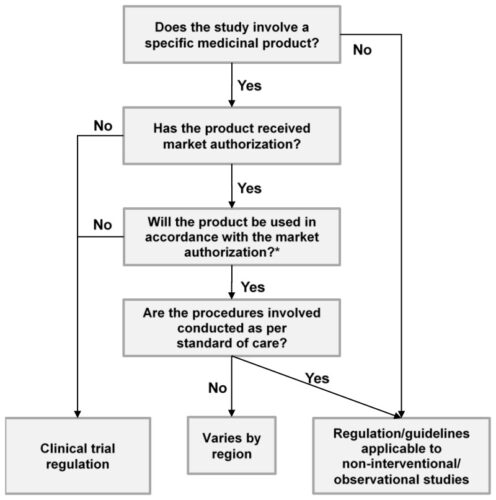
The chart below shows how a non-interventional observational study is different from a clinical trial.
Figure 1: Classification of a non-interventional/ observational study (Source: https://www.ncbi.nlm.nih.gov/books/NBK493601/)
Do post-approval studies require an insurance policy to protect patients from potential damage?
No. Unlike clinical trials, post-approval studies do not require a specific study insurance policy to protect participants from injuries or harm.
The reason why post-approval studies do not need insurance coverage is that in these types of studies patients are not exposed to the risks of unapproved experimental compounds.
Is a post-approval study more expensive than a clinical trial?
As a general rule, post-approval studies are cheaper to execute compared to clinical trials.
Clinical trials involve more risks for participants and require closer patient follow-up and data monitoring, which increases the overall cost of the study.
What types of post-approval studies are there?
Post-approval studies can be divided into two basic groups: retrospective and prospective.
In retrospective studies the period of time observed is that prior to the enrollment of a participant in the study.
On the other hand, a prospective study observes events that take place in the present and in the future, after a patient has been enrolled in the study.
There is yet a third possibility: an ambispective study, which evaluates events both retrospectively and prospectively.
Can a post-approval study be conducted in any country of Europe?
Yes. Post-approval studies are common practice all over Europe.
Each European member state may have its own national legislation but the requirements to carry out these studies are very similar from country to country.
In the lines below we will focus on the particular aspects of conducting a post-approval study in Spain, as an example of an EU country.
What are post-authorization safety studies (PASS) in Europe?
European guidelines [1] define post-authorization safety studies (PASS) as follows:
“A post-authorisation safety study (PASS) is a study that is carried out after a medicine has been authorised to obtain further information on a medicine’s safety, or to measure the effectiveness of risk-management measures. The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) is responsible for assessing the protocols of imposed PASSs and for assessing their results.
The purpose of the information in PASSs is to evaluate the safety and benefit-risk profile of a medicine and support regulatory decision-making. They aim to:
- identify, characterise or quantify a safety hazard;
- confirm the safety profile of a medicine, or;
- measure the effectiveness of risk-management measures.
PASSs can either be clinical trials or non-interventional studies.
PASSs are either imposed or voluntary.
Marketing-authorisation holders (MAHs) are obliged to carry out imposed PASSs. These include studies that are a specific obligation for a marketing authorisation granted under exceptional circumstances and other studies that the PRAC requests the company carry out.
Voluntary PASSs are sponsored or conducted by MAHs on their own initiative. They include non-imposed studies that are requested in risk management plans.”
Where can I download a template for a post-authorization safety study protocol?
The European Medicines Agency (EMA) has published this Guidance for the format and content of the protocol of non-interventional post-authorisation safety studies [2].
What is the legislation governing the conduct of post-approval studies in Spain?
Postmarketing studies in Spain are regulated by the SAS/3470/2009 Order, of September 16th [3], containing the guidelines for post-authorization studies of observational nature, for medicines of human use.
What are the administrative procedures to open a post-approval study in Spain?
Four main procedures are involved in the activation of a post-approval study in Spain: regulatory classification, ethics approvals, autonomous community approvals, and site contracts.
Firstly, all post-approval studies in Spain must be submitted to the national regulatory authority, namely Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) for study classification. This is not an authorization as such, but rather a classification confirming the type of study to be performed. This classification by AEMPS may take 2-4 weeks.
Secondly, once the study has been classified by AEMPS, the protocol and the rest of documents (patient information sheet, informed consent, etc.) must be submitted to the required ethics committees.
Officially speaking, only one ethics committee approval should be sufficient in a multicenter post-approval study. However, the reality is that at least 30% of the ethics committees of the participating sites will finally require a full formal evaluation of the study documentation. Therefore, in multicenter studies there will be more than one ethics approval.
Thirdly, in addition to the ethics committee approvals, post-authorization studies with prospective follow-up in Spain have the particular requirement of an additional authorization issued by each involved Autonomous Community (provinces). These are regional approvals issued by authorities of each province covering the sites located in that specific region.
Finally, another administrative procedure involved before site activation is the negotiation of site contracts. In a postmarketing study in Spain, every single hospital will require a written contract between the sponsor and the site. The hospital contract units will normally facilitate a study contract template to be filled out by the sponsor or the appointed clinical research organization (CRO).
What are the ethical aspects to be considered in post-approval studies in Spain?
For a post-approval study to be ethically justifiable it must be well designed and comply with the basic ethical principles contained in the Declaration of Helsinki of the World Medical Association (WMA) on ethical principles for human medical research, and in its subsequent revisions.
All post-authorization observational studies should be evaluated by an accredited ethics committee.
In all postmarketing studies, a patient information sheet and informed consent form (PIS/ICF) must be used so that patients can give their written consent to participate.
In retrospective studies, if patients are dead or unreachable, their data and biological samples could still be used with the permission of the ethics committee. The recommendation is that the principal investigator documents evidence of his/her efforts to obtain the consent.
When the subjects are minors or incapacitated, informed consent will be requested of legal guardians.
The sponsor and the investigators of the study must guarantee the confidentiality of patient data and ensure that the provisions of the applicable data protection laws are complied with at all times.
In Spain, applicable personal data protection laws include the General Data Protection Regulation (GDPR) and the Spanish Organic Law 3/2018, of December 5th, of Personal Data Protection and the guarantee of digital rights.
Post-authorization observational studies are exempt from the obligation to have an insurance policy.
In the case of a post-authorization study with prospective follow-up, the clinical protocol should describe the procedures that will be used to ensure that the study will not modify the prescription habits of the doctor, or the dispensing habits of the pharmacist.
The prescription of the medicine will have to follow the usual channels of standard of care.
Researchers will need to ensure that their participation in the study does not interfere with their patient care duties.
Researchers may receive compensation proportional to the time and additional responsibilities dedicated to the study, without prejudice to the internal regulations of the institutions employing the researchers.
The financial aspects of the study (budget) must be explicit and transparent and must be submitted to the ethics committee evaluating the study.
Participation in the study (by both investigators and subjects) must be free and voluntary.
What aspects are to be considered regarding the storage of documents in a post-approval study in Spain?
The documentation related to post-authorization studies will be included in the study master file and will consist of the essential documents needed for the evaluation of the procedures of a post-authorization study and the quality of the data obtained. These documents must demonstrate compliance by the researchers and sponsor of the established requirements for post-authorization studies.
The post-authorization study master file will provide the basis for the audits that the sponsor can carry out through independent auditors and/or inspections of the competent authorities.
The sponsor and the researchers will keep the essential documents and materials of each study for at least five years (according to Spanish law) after completion of the study, or for a longer period if other applicable requirements apply.
Essential documents and materials should be archived so that they can be
easily made available to the competent authorities, if requested.
Are post-approval studies audited by authorities in Spain?
Yes. The competent health authorities of the Spanish Autonomous Communities, within the scope of their competencies, will verify compliance with the legal requirements related to post-authorization studies carried out in Spain, through the corresponding inspections and in accordance with established procedures.
The inspections can be carried out before, during or after the completion of the study by duly qualified inspectors.
Inspections may be conducted in the hospitals in which the study is carried out, in any laboratory or center used, in the facilities of the sponsor and/or the organizations or research companies involved by contract in the study, and in the ethics committee that evaluated it.
Who can help me manage a post-approval study in Spain?
Most Clinical Research Organizations (CROs) in Spain are capable of providing regulatory, administrative, and clinical operations support to conduct post-approval studies in Spanish hospitals.
Conclusion
Post-approval studies are important to obtain additional safety and efficacy data, after a drug has been approved. Europe, and particularly Spain, is a convenient territory to execute these types of studies.
We finally recommend the video below about post marketing surveillance studies by Thomas Walther.
You can contact us at info@sofpromed.com if you need help to carry out a post-approval study in Europe.
References:
[1] Post-authorisation safety studies (PASS)
[3] Orden SAS/3470/2009, de 16 de diciembre