For more information about the Viedoc EDC solution, please contact us at info@sofpromed.com
Oncology clinical trials are complex and require the use of effective Electronic Data Capture (EDC) tools to ensure high-quality data collection, cleaning, and analysis.
As a cancer expert CRO, Sofpromed has adopted the Viedoc EDC solution to manage clinical data in phase I-IV oncology studies, particularly in the area of solid tumors (e.g. soft tissue sarcomas, gynecologic cancers, among others).
Viedoc has greatly empowered and streamlined Sofpromed’s oncology-focused data management and statistical programming services, increasing the value offered to clinical trial sponsors developing anticancer drugs.
Why is Viedoc an ideal EDC software for cancer trials?
Let’s consider 13 reasons.
1. Accurate Capture of Drug Efficacy Data
Viedoc enables the implementation of disease-specific forms to accurately capture drug efficacy data typically involved in cancer trials. For example, in solid tumor studies, a tumor assessment form is required to collect radiological evaluation information obtained from CT scans and MRIs. In these trials, radiological tests are usually performed at baseline, and every 6 or 8 weeks to measure tumor size (according to RECIST criteria) and density (according to Choi criteria). This is done to evaluate the radiological response of the tumor (complete response, partial response, stabilization, or progression), which indicates whether the tumor mass has shrunk, grown, or remained the same. The date of tumor progression (growth or appearance of new lesions) is used to measure progression-free survival (PFS). In addition, overall survival (OS) is another important efficacy endpoint measured by using survival follow-up forms.
2. Accurate Capture of Drug Safety Data
Drug-related toxicities are very important in oncology trials, and particularly in phase 1 dose escalation studies in which drug safety is one of the main endpoints. By using Viedoc, adverse event forms can be easily implemented to register treatment-emergent adverse events (TEAEs) —linked to the corresponding concomitant medications, if any— which can be classified according to the Common Terminology Criteria for Adverse Events (CTCAE), a product of the US National Cancer Institute (NCI).
3. Data Management for Oncology Studies of Any Phase and Size
The Viedoc EDC software is a flexible and convenient tool for the collection and management of data in cancer clinical trials of any phase (1, 2 or 3) as well as in observational or post-approval studies (non-interventional). It can also be used to create regional, national, or international cancer registries. The system is scalable and able to handle not only the specific needs of small early-stage safety-focused studies, but also the large number of patients and sites needed in international pivotal or registration trials.
4. Easy and Fast Study Setup
The Viedoc EDC suite has been designed to facilitate the implementation of electronic case report forms (eCRFs) through a highly intuitive study set up interface. This allows data managers to build data forms quickly and effortlessly, thus shortening eCRF set up timelines for sponsors.
5. A Beautiful Interface for Data Entry
A unique feature of the Viedoc EDC system is its wonderful look and feel. Study coordinators and data entry staff at clinical sites will appreciate the experience of using Viedoc’s clear and good looking screens, which will make their daily data entry and query resolution tasks more pleasant.
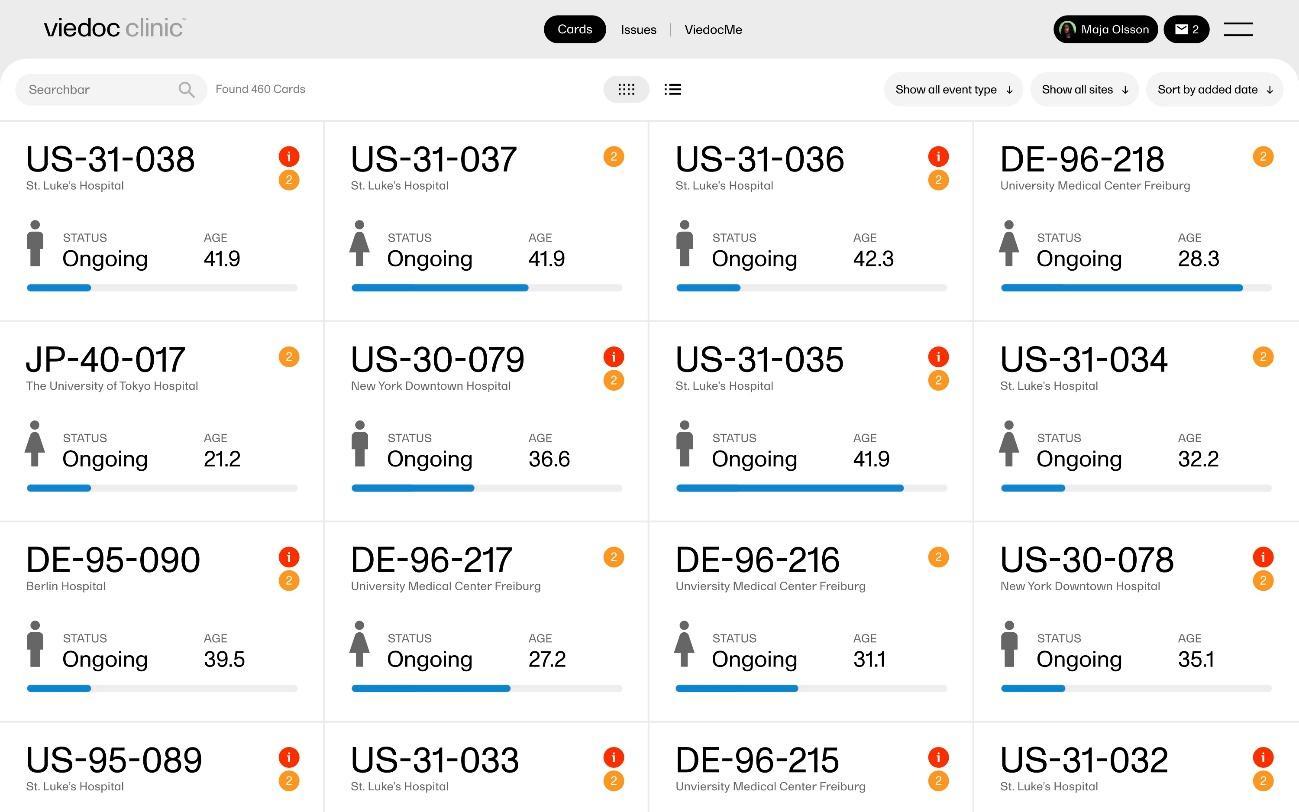
Figure 1: Viedoc EDC Patient List View
6. A Highly Intuitive, Easy-to-Use Tool
The Viedoc EDC software stands out for its intuitive design and unique ease of use. Smartly designed to make life easier, Viedoc users will realize how easy it is to navigate through the data forms to enter data and solve queries. Clarity and simplicity are at the core of the Viedoc ecosystem.
7. A Guided Workflow for Different Study Roles
Viedoc permits a variety of user roles (e.g. investigator, data manager, monitor) providing useful role-specific help messages to facilitate the navigation and tasks of these users. Each person is guided to easily handle signatures in a form, query resolution, and missing data completion, among other tasks.
8. e-Tablet Support
One of Viedoc’s added values is its ability to support multiple platforms, including e-tablets. Hospital personnel and patients (when providing patient-reported outcomes, ePRO) will enjoy Viedoc EDC’s tablet-adapted interface for on the go use. With regard to cancer trials, the use of e-tablets becomes relevant in those trials collecting ePRO, such as patient-reported quality of life, pain, and information on analgesic use.
9. Powerful Real-Time Metrics Panel
Considering that complete and accurate data is fundamental for successful clinical trials, the Viedoc EDC platform provides a powerful, real-time metrics panel to track different study parameters at study, country or site level. Among other aspects, this allows tracking the number and status of data queries.
10. Data Review and Cleaning Capabilities
The Viedoc EDC solution integrates potent data review and query management functionalities that accelerate the overall data cleaning process. For example, the system allows for role-based query management and monitors can perform selective source data verification (SDV) on item level. Data locks can be executed on form, visit, patient, and study levels.
11. Randomization Features
Viedoc is the perfect tool to manage randomized oncology trials. The system permits the creation of pre-computed static lists and dynamically generated randomized lists. Through the use of an additional module (Viedoc Logistics), trial supply management functions can be fully integrated in the EDC system to control study drug allocation as well.
12. Data Exports in Different Formats
Viedoc facilitates the work of statistical programmers by providing data exports in Excel, CSV, SAS, PDF / A (compliant to FDA submission, eCTD), and CDISC ODM formats. Data exports can be manually performed at any time, and they can be pre-scheduled.
13. Full Compliance with International Regulatory and Data Protection Standards
The Viedoc set of software tools is fully compliant with industry standards, including the regulatory requirements of FDA, EMA, JPMA, and CFDA, as well as data protection laws such as the GDPR (Europe), APPI (Japan), HIPAA (United States), and PISS (China). In addition, the software contains an audit trail module and manages electronic signatures according to FDA 21 CFR part 11.
Conclusion
Managing clinical data in cancer studies can be challenging and trial sponsors should use effective EDC tools to guarantee timely and accurate data capture, cleaning, and analysis. As an expert oncology CRO, Sofpromed can provide high-quality data management and statistical programming services based on the powerful features of the Viedoc EDC suite at affordable cost.
For more information about the Viedoc EDC solution, please contact us at info@sofpromed.com