Check the Clinical Trial Cost Calculator here and calculate your trial budget in seconds!
Please contact us and we will send you a ballpark clinical trial quotation within 48 hours.
Click here if you want to download a full clinical trial quote in PDF
Biotechnology and pharmaceutical companies need to know the cost of a clinical trial to plan the funding of their drug development programs.
In particular, U.S. biotech companies seeking to have their drug candidates approved by the Food and Drug Administration (FDA) need to be aware of the cost of conducting clinical trials in American clinical sites.
However, despite the critical importance of knowing the clinical trial bill, biotech companies in the early stages of drug development may have a difficult time to have a clear picture of all the costs involved in a clinical study.
Clinical Research Organizations (CROs) are companies specialized in managing clinical trials from beginning to end, and they can provide detailed clinical trial quotations to biotech firms, including all the expenses involved in a phase I, II or III study.
Therefore, biotech managers can get support from CROs to calculate how much their clinical trials will cost, including both CRO services and pass-through (third party) fees.
This article seeks to help biotech chief executive, operations, and financial officers in understanding the actual costs involved in the execution of a clinical trial.
If you want to receive a quick clinical trial quotation now via email, please contact us here.
What is a Clinical Research Organization (CRO)?
If you need a clinical trial quote, a Clinical Research Organization (CRO) is the type of company that can help you.
A CRO is a company dedicated to managing clinical trials taking care of all or several tasks involved in a clinical study, including regulatory affairs, site selection, onsite monitoring, data management, biostatistics, and medical writing, among others.
Since CROs have a global view of all the stages and elements of a clinical study, they are able to provide detailed budgets of all clinical trial expenses.
What is the cost of a clinical trial?
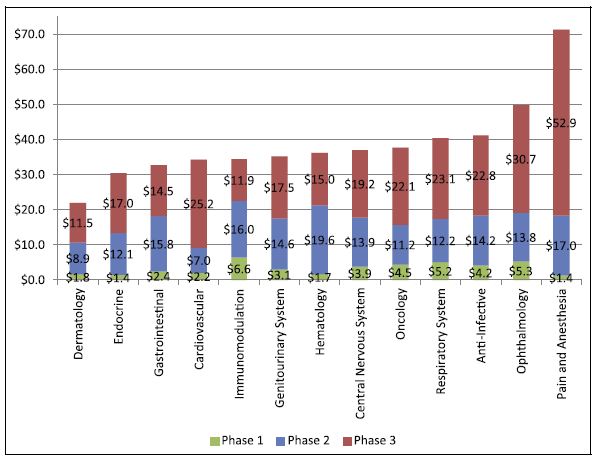
The costs of phase 1, 2, and 3 clinical trials conducted in the United States are in the range of US$1.4-6.6, 7.0-19.6, and 11.5-52.9 million respectively.
These numbers were calculated by Aylin Sertkaya et al. in their excellent article about key cost drivers of pharmaceutical clinical trials in the United States [1].
In any case, estimating the actual price of a clinical study is not easy given the various factors involved.
Let’s analyze these factors.
What are the main cost drivers in a clinical trial?
The total cost of a clinical trial depends on many variables, but the main cost factors are:
(1) the number of participants, and
(2) the complexity of the clinical trial protocol.
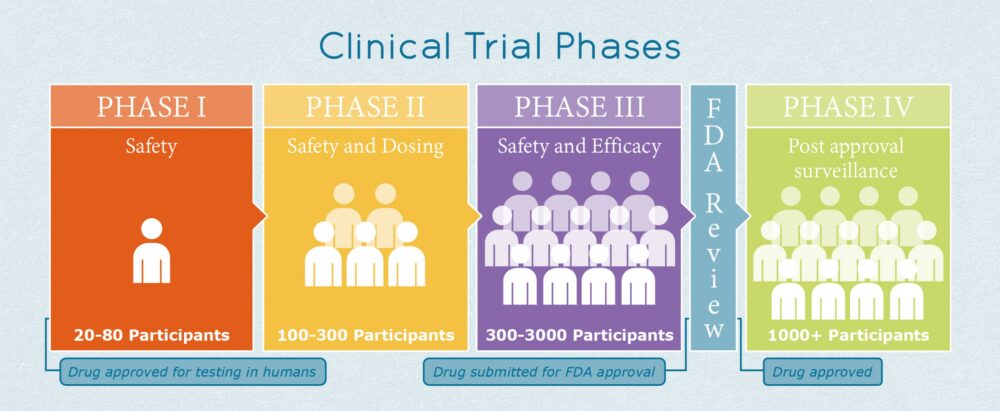
Firstly, the number of participants in a clinical trial is highly related with the study phase.
For instance, phase 1 clinical trials —which are focused on evaluating drug safety— recruit an average of 20-80 patients.
Phase 2 trials —which analyze safety, dosing, and preliminary efficacy— normally include between 100 and 300 participants.
Later stage phase 3 clinical trials are larger studies assessing safety and efficacy and can enroll several patients depending on the therapeutic area (between 300-3,000 participants), as shown in Figure 1 below.
Figure 1: Clinical Trial Phases [2]
The number of participants in a clinical trial is a key overall cost driver because each recruited patient implies a fee to be paid by the Sponsor to the hospital, in order to cover medical tests and procedures.
Secondly, the complexity of the clinical trial protocol also has a direct impact on the trial cost.
For example, a long and complex cancer treatment —that may include several intravenous drug administration cycles up to disease progression— will involve several patient visits to the clinical sites, with the corresponding clinical tests (e.g. CT scans, ECG), which will significantly increase the expenses of the study.
On the other hand, a simple and short clinical study with few visits using an oral drug may include less per-protocol visits and tests, which will lower the site’s bill.
Therefore, the clinical complexity and duration of a trial play a decisive role in the price of a clinical study.
In addition, there are other clinical trial cost factors like the technological tools and applications to be used, the number of external vendors needed (e.g. central laboratories and drug depots), and the countries in which the research is carried out.
Is conducting a clinical trial in the United States more expensive than doing it in other parts of the world?
In general yes. Conducting a clinical trial in the United States of America involves higher costs compared to running a study in other countries (e.g. Europe or Asia).
How much do phase 1 clinical trials cost?
The average cost of a phase 1 study conducted at a United States clinical site ranges from US$1.4 million to US$6.6 million, including estimated site overhead and monitoring costs [1].
How much do phase 2 clinical trials cost?
A phase 2 study in the U.S. costs from US$7.0 million (cardiovascular) to US$19.6 million (hematology) [1].
How much do phase 3 clinical trials cost?
Phase 3 clinical trials in the United States (many of them considered “pivotal”) range from US$11.5 million up to US$52.9 million [1].
Figure 2: Clinical Trial Cost by Phase and Therapeutic Area [1]
In another study performed by Thomas J. Moore et al [3], the estimated cost of pivotal clinical trials supporting the FDA approval of 101 approved drugs was a median of US$48 million (IQR US$20 million–US$102 million).
Each individual pivotal trial cost a median of US$19 million (IQR US$12 million–US$33 million).
The estimated cost per patient was US$41,413 (IQR US$29,894–US$75,047), and each patient visit to the study clinic cost an estimated median of US$3,685 (IQR US$2,640–US$5,498).
How are clinical trial quotations normally structured by CROs?
CROs structure their quotations in different ways, but normally they divide their budgets in two main categories: (1) CRO services, and (2) pass-through costs.
The CRO service category typically includes concepts such as:
- Regulatory affairs
- Site identification and selection
- Site contracting and payments
- Site initiation and activation
- Site management
- Onsite monitoring
- Drug safety management
- Drug logistics
- Biological sample logistics
- Clinical supplies logistics
- Medical writing
- Site close-out
- Project management
- Study files / document management
- Data management
- Statistical programming
- Quality control
The pass-through costs category normally includes the following items:
- Shipping costs
- Clinical supplies and packages
- Office supplies
- Fee per patient to be paid to the clinical site
- Publication fees
- Ethics / Regulatory agency evaluation fees
- Site contract fees
- Travel costs
- Drug distribution services
- Central laboratory services
- EDC system for data collection
- IRT system for drug management
- Radiological imaging platforms
- Electronic Trial Master File (eTMF)
- Drug safety platform
What are the more costly line items in a CRO clinical trial quote?
The more costly items in a clinical trial quote are site management and project management costs (CRO direct services). In addition, the most significant pass-through expense is the fee to be paid to the clinical sites for each recruited patient (i.e. costs of clinical procedures and tests).
How much do hospitals charge for each patient treated in a clinical trial?
The amount of money to be paid by the Sponsor to the clinical sites in which the patients are treated —to cover medical procedures and tests— varies across study types, but in the U.S. this can go from US$20,000 up to US$70,000 per patient.
How much does an electronic data capture (EDC) system cost?
In a clinical trial, an EDC system is a tool used by clinical sites to enter clinical data.
The cost of an EDC software —license and hosting price— is in the range of US$1,000 and US$5,000 per month of usage, depending on the type of solution.
This price does not include the initial services required to design, document, implement, test, and validate the EDC tool.
Please read the following article to learn more about EDC software costs: How Much Does an Electronic Case Report Form (eCRF) Cost?
How much does it cost to manufacture a drug for a clinical trial?
The answer to this question depends on many factors, but usually the manufacturing of a drug product batch for a clinical trial may be in the average range of US$300,000 to 1 million.
This does not include the cost of producing the drug substance or active pharmaceutical ingredient (API).
Please read the following article to learn more about drug manufacturing costs in the context of clinical trials: How Much Does it Cost to Manufacture a Drug for a Clinical Trial?
How much does it cost to pack, label, store, and distribute a drug in a clinical trial?
The cost of packaging, labeling, storing, and distributing a drug in a clinical trial may be in the range of US$40,000 to 100,000 per study.
The cost of managing drugs in a clinical trial depends on the quantity of drug required, the number of hospitals, the temperature conditions, the number of shipments from source to depot, and the number of shipments from depot to sites.
Please read the following article to obtain more information about drug packaging, labeling, storage, and distribution costs in the context of clinical trials: What is the Cost of Packaging, Labeling, Storing, and Distributing Drugs in a Clinical Trial?
Conclusion
The costs of phase 1, 2, and 3 clinical trials conducted in the United States are in the range of US$1.4-6.6, 7.0-19.6, and 11.5-52.9 million respectively.
A CRO can help you come up with a more precise calculation for your specific clinical trial.
Please contact us and we will send you a ballpark clinical trial quotation within 48 hours.
Click here if you want to download a full clinical trial quote in PDF
References: