Are you a biotechnology or pharmaceutical company looking for a clinical research organization (CRO) partner specialized in ovarian cancer clinical trials in the United States?
Sofpromed is an oncology-focused full service CRO specialized in the management of clinical trials in ovarian cancer, among other therapeutic areas, in the US. Our team comprises qualified and competent professionals with long-standing experience in assisting emerging biotech companies in the conduct of clinical research on new drugs, therapies, and approaches to combat different types of cancer throughout the country.
- $What Is Ovarian Cancer?
- $Which Women Are Most at Risk for Ovarian Cancer?
- $What Are the Main Therapies and Treatments for Patients with Ovarian Cancer?
- $Great Commitment and Oncology Expertise for Ovarian Cancer Clinical Trials
- $What Services Does Sofpromed Provide?
- $Sofpromed: An Ideal Partner for Conducting Ovarian Cancer Clinical Trials
What Is Ovarian Cancer?
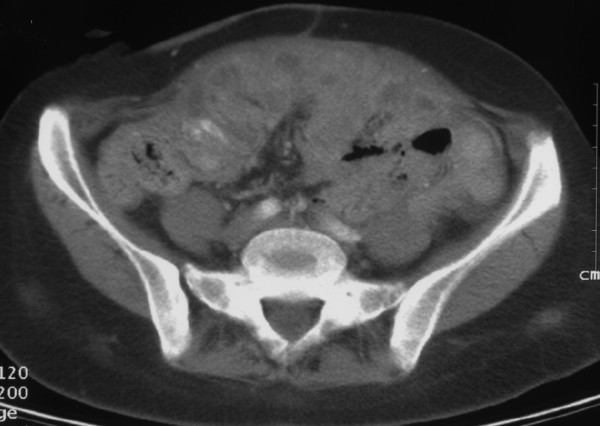
Like all cancerous diseases, ovarian cancer appears when malignant cells in the body start to replicate out of control. In particular, this type of cancer affects the female reproductive system. Other gynecological cancers include cervical, endometrial or vaginal cancers, among others.
Image 1. Female reproductive system with ovarian cancer
In the past, ovarian cancers were believed to start only in the ovaries, yet recent research has shown that this disease may certainly begin in the fallopian tubes. Moreover, the ovaries are composed mainly of 3 types of cells, each of which can potentially develop into a different kind of tumor: [1]
- Epithelial ovarian carcinomas: they start in the cells covering the outer surface of the ovary, and represent about 85% to 90% of the total ovarian cancers.
- Germ cell tumors: these tumors start in the reproductive cells, that is, the woman’s ‘eggs’ (ova). Unlike the other types of ovarian cancer, young women —mainly teenagers or women in their twenties— are more at risk for developing germ cell tumors.
- Stromal cell tumors: formed in the tissues which hold the ovaries, this is the least common type of ovarian cancer, and it is usually detected in the early stages.
Ovarian cancer is not one of the most frequent cancers, which explains why there is not much research done on it. Despite this, it is important to keep in mind that ovarian cancer is an extremely aggressive cancer that is often diagnosed at an advanced stage.
In actuality, only 47.6% of women with ovarian cancer survive for five years or longer. This results from the fact that these cancer cells spread very rapidly to other parts of the body, what is known as ‘metastasize’; as a consequence, they can even invade and/or destroy nearby tissues and organs.
Today, nearly 70% of patients with epithelial tumors are diagnosed when the survival rate is only 30%. Therefore, more attention must be focused on new treatments to enhance the life expectancy of women who have this serious illness or, at least, reduce their suffering.
Which Women Are Most at Risk for Ovarian Cancer?
A woman’s lifetime risk of getting ovarian cancer is about 1 in 78. However, in comparison with other types of cancer that affect women, the survival rates of people with ovarian cancer are considerably low. Actually, this type of cancer is the fifth most common cause of cancer death in the United States.
For example, according to the American Cancer Society, an estimated 19,880 women will be diagnosed with ovarian cancer in the US in 2022, of whom 12,810 will eventually die from the disease. [1]
Although most women who develop ovarian cancer are not in a high-risk group, there are a number of factors which may increase the probability. These risk factors include:
- Being middle-aged or older
- Having had ovarian, breast, or colorectal cancer
- Having a family history of the abovementioned cancers
- Being overweight or obese
- Having never given birth or having had problems getting pregnant
- Using fertility treatment with in vitro fertilization (IVF)
- Giving birth after 35 years of age
- Taking hormone therapy after menopause
In addition, there are several factors which may prevent the risk of ovarian cancer. For instance, women are less prone to get ovarian cancer when they are pregnant, breastfeeding, or when they have recently given birth.
Moreover, both the surgery to remove the ovaries and fallopian tubes (known as ‘bilateral salpingo-oophorectomy’) and the use of birth control pills have also been associated with a lower risk of ovarian and breast cancer. In fact, the preventive effects of contraceptive pills remain for several years after a woman stops taking the pill. [2]
There are also certain factors with unclear effects on this kind of cancer. These factors have to do with androgens, talcum powder, and a high fat diet. Nevertheless, more detailed and qualitative investigation is required to determine whether or not these elements have real effects on ovarian cancer.
Furthermore, it should be highlighted that not all women are equally prone to ovarian cancer, nor does this disease affect all women in the same way. Old women, particularly those over the age of 63, constitute the most vulnerable high-risk group. Because of this reality, they should be a target of current prevention efforts and potential treatment options.
In terms of race or ethnic origin, the prevalence and effects of ovarian cancer also appear to differ markedly from one woman to another. Statistics show that white women are more likely than black women to get ovarian cancer. As an illustration, while the incidence rate for white women is 14.1 cases per 100,000 women, the prevalence rate for African-American women represents 10,1 cases per 100,000. [3]
Nonetheless, the life expectancy for black women is considerably lower than it is for white women in the same situation. By way of comparison, a 2019 review found that African-American females with ovarian cancer were 18% more likely to die from this cancer than Caucasian ones. [4]
This significant disparity may be due to social, cultural or economic reasons since, historically, black women have been an underprivileged group in the United States. As a result, this has made it difficult for them to have access to healthcare. This is evidenced by the fact that, at least until recently, African American women with ovarian cancer were diagnosed at a later stage and, as a consequence, they were 25% less likely to receive medical treatment than white women. [4] Fortunately, their situation is improving, albeit at a slow pace.
What Are the Main Therapies and Treatments for Patients with Ovarian Cancer?
As already stated, ovarian cancer is generally diagnosed late, but there are some signs that should be paid attention to in order to detect and respond to this insidious disease at the earliest possible time.
Ovarian cancer can manifest in many different ways. Its symptoms range from feeling full quickly after eating and a frequent need to urinate to constipation, difficulty eating, abdominal bloating, pain or discomfort in the pelvis, among others.
Although these symptoms are also characteristic of other conditions —such as colon cancer, twisted ovaries or endometriosis— they should not be disregarded. In fact, it is very important to know how to identify ovarian cancer symptoms and seek medical advice if they appear. After all, the earlier cancer is diagnosed, the more treatable.
And what measures and treatments have to be followed after ovarian cancer has been diagnosed? There are two main types of treatment used for this gynecologic cancer:
- Surgery: the kind of operation will depend on the type of cancer as well as its stage. Patients may have surgery to remove either the womb or the ovaries and the fallopian tubes.
- Chemotherapy: it consists of applying a series of medicines to kill cancer cells. The most commonly used chemotherapy to treat ovarian cancer is called carboplatin.
Alternative treatments to treat ovarian cancer include:
- Radiotherapy: this type of therapy aims to shrink the size of ovarian cancer, lessen the symptoms in patients with advanced disease, and prevent the disease from coming back.
- Targeted medicines: these drugs target cancer cells’ inner processes and attack them to minimize the risk of complications.
- Hormone treatments: the objective of these therapies is to block the production of the hormone estrogen to prevent further growth and spread of ovarian cancer.
Certainly, it is difficult to predict exactly the most appropriate ovarian cancer treatment for each patient. It will largely depend on factors such as the size and kind of ovarian cancer, the phase of the disease, the age of the patient as well as their overall state of health.
Moreover, bearing in mind that no two patients are completely alike, responses to treatment may also vary considerably between seemingly similar women. For this reason, only a gynecologic oncologist is properly trained to determine the best treatment options based on each woman’s individual situation.
Gynecologic oncologists have completed obstetrics and gynecology residency programs, as well as three to four additional years of intensive training about surgical, chemotherapeutic, radiation, and research techniques.
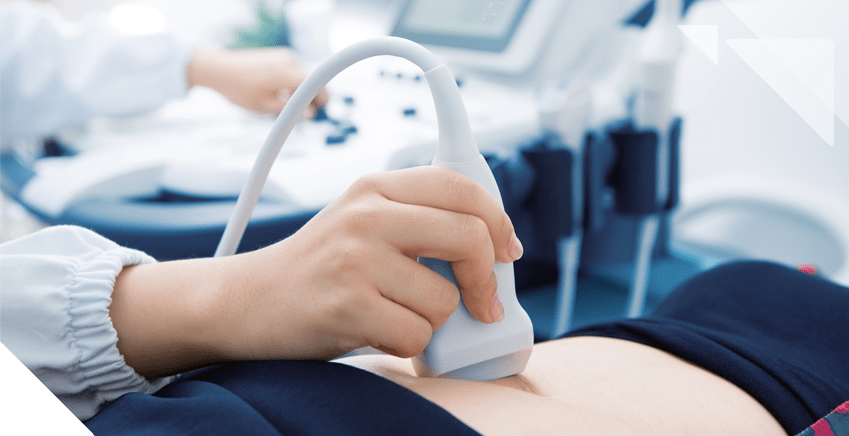
Thanks to their broad knowledge and expertise, these licensed medical professionals should be consulted for diagnosis and treatment of gynecologic cancers, among which is ovarian cancer. They will take the time needed to explain the treatment options and processes —as well as their benefits, risks, and side effects— both to the patients and their families. In addition, they will examine the patient and work with her to create the most adequate treatment plan and provide the best care for her specific condition.
Image 2. Gynecologic oncologist performing an ultrasound examination
Sometimes, however, it may not be possible to cure ovarian cancer, especially when the disease is at an advanced stage. In those cases, the doctor will prescribe medicines and treatment measures focused on limiting the cancer, as well as its symptoms, and thus help patients live longer.
Furthermore, it may be helpful to seek a second opinion prior to the beginning of the treatment. It consists of getting an opinion from two or more cancer specialists, and it is particularly recommended if your cancer has multiple treatment options or if the patient is in a critical condition. Not only is a second opinion useful to confirm the accuracy of the diagnosis, but it can also alter the treatment plan and make sure that the most effective treatment is received.
Also noteworthy is the importance of clinical research in the development of new drugs and treatments to combat ovarian cancer. Managing a trial in this illness presents particular operational challenges. This is due in part to the fact that few clinical studies have been conducted on ovarian cancer in comparison to other more common types of cancer and, as a result, the study of this disease remains a relatively unexplored field.
For this reason, in order to address these challenges, it is highly advisable to have the services provided by a CRO specialized in oncology clinical trials, which will ensure the successful conduct of studies from an operational standpoint.
Great Commitment and Oncology Expertise for Ovarian Cancer Clinical Trials
Sofpromed has significant expertise in the handling and management of clinical trials in multiple cancerous diseases. One of the areas in which we are specialized is gynecologic oncology and, more precisely, we are experts in managing studies on ovarian and endometrial cancers.
Our CRO stands out for its ability to offer a “full service strategy”. This means that sponsors can delegate to our staff all the tasks and responsibilities involved in the conduct of a clinical study. In this way, they can focus on the main business operations.
Since its foundation in 2012, Sofpromed has provided its services worldwide. In particular, most of the biotechnology and pharmaceutical companies to which we provide assistance are located in Europe and the United States.
Actually, an increasing number of clients rely on our services due to the work quality, commitment and motivation of our team. The advantages and benefits of using our services are described below.
Expertise and senior profile of our personnel
Sofpromed’s team is composed of experienced clinical research associates (CRAs), data managers, statistical programmers and other professionals specialized in oncology.
It is important to emphasize that the clinical operations staff we provide to manage clinical trials have, on average, ten years of experience supporting biotech and pharma companies planning to conduct clinical trials in the United States.
Access to KoL (key opinion leaders)
Moreover, our company can provide access to key opinion leaders (KoL), that is, to trusted, well-respected experts in the area of oncology. Indeed, by virtue of our expertise in oncology and our collaboration with international specialists, we already have an extensive network of contacts, including investigators and medical oncologists in the US.
Not only do we offer proficient health care professionals, but we are also familiar with some of the most prestigious hospitals recruiting patients in cancer. Therefore, we can provide biotech companies with the top sites to run a clinical trial.
Responsiveness and client proximity
As a small CRO, we can be very responsive. Many times, small biotech companies do not get good services from multinational CROs. For this reason, our CRO is their perfect match. In fact, our profound understanding of their needs and challenges, as well as the proximity offered to our clients, makes us a real partner.
What Services Does Sofpromed Provide?
Sofpromed provides a comprehensive range of services to help improve the execution of clinical research activities. By extension, our assistance is intended to enhance the efficacy of current treatments as well as the quality of information collected during the course of clinical investigation, from study initiation to close-out.
Our CRO service list includes:
- Clinical operations: these services are the glue between stakeholders. Some examples include site selection and activation, clinical site management, onsite monitoring, drug safety monitoring, and many others.
- Biometrics: especially data management and SAS statistical programming.
- Web tools: we provide mainly three of them, the Electronic Data Capture (EDC) system, the electronic Trial Master File (eTMF) and the DICOM imaging platform for radiological reviews.
The quality of these services plays a major role in accelerating all the processes related to the execution of clinical trials. Sofpromed offers this support to accomplish its prime objective: to ensure that all the needs of small-sized biotech companies and trial sponsors are successfully fulfilled.
Sofpromed: An Ideal Partner for Conducting Ovarian Cancer Clinical Trials
Sofpromed is the ideal partner for small-sized biotech companies developing anticancer drugs. Our CRO is composed of qualified and experienced clinical operations, biometrics and upper management teams that stand out for their great responsiveness, firm commitment to research as well as their willingness to collaborate with medical professionals, in both commercial and academic studies.
Thanks to our experience and clear understanding of the specific needs of biotechnology start-ups involved in the oncology space, we can provide high-quality services aimed at meeting, in an efficient way, all the challenges and demands of today’s scientific environment, characterized by rapid and complex clinical research and technological development.
If you need a CRO to conduct an ovarian cancer clinical trial in the US, please contact us at info@sofpromed.com
References:
[1] American Cancer Society. 2022. “Ovarian Cancer: Key Statistics for Ovarian Cancer.” [Accessed 6 April 2022]
[2] -. 2022. “Ovarian Cancer Risk Factors.” [Accessed 8 April 2022]
[3] Moorman, Patricia G., Rachel T. Palmieri, Lucy Akushevich, Andrew Berchuck, and Joellen M. Schildkraut. 2009. “Ovarian Cancer Risk Factors in African-American and White Women.” American Journal of Epidemiology. [Accessed 11 April 2022]
[4] Burgess, Lana. 2021. “Ovarian Cancer Statistics”. Medically reviewed by Amy Tiersten, MD. Medical News Today. [Accessed 11 April 2022]