Dr. Manoj Jadhav, PhD, FCP is a Translational Clinical Pharmacologist and the Founder and CEO of ISHA Therapeutics, a New Jersey-based company specialized in supporting biotech and pharma companies in their early stage clinical drug development programs.
In this dialogue we will speak about key regulatory aspects of early clinical development, with the aim of providing useful insights and recommendations for biotech companies developing new drugs in the United States and planning interactions with the Food and Drug Administration (FDA).
Patricio Ledesma (PL): Hello Manoj. Thank you for your availability to talk about regulatory affairs in early clinical development programs.
Manoj Jadhav (MJ): Thank you Patricio for this unique opportunity to interact with you and share my experiences and understanding on drug development and regulatory strategies for successful development and approval of safe and efficacious drugs.
PL: Could you tell us about your professional expertise and main areas of scientific knowledge?
MJ: I studied in India at prestigious universities in Pune, Delhi, and Mumbai, and received my Bachelors, Masters, and a Ph.D. degree, all in Pharmacy. Throughout the years, I had excellent opportunities to research and develop liposomal, transdermal drug delivery systems, testing their efficacy in-vitro and in-vivo. The turning point for me was during my Ph.D., when I helped develop the first Indian liposomal amphotericin B. This led to two randomized, controlled clinical studies, one in HIV/AIDS patients with cryptococcal meningitis as a systemic fungal infection and one in patients with leukemia.
Through my research on liposomal amphotericin B, I gained enough exposure to both pre-clinical and clinical development to begin working as an assistant professor at the Infectious Diseases Department of KEM Hospital, a tertiary care center in Mumbai, where I got involved in teaching and guiding students from diverse backgrounds to pursue degrees in pharmaceutical medicine. Soon after, I moved to the United States as a post-doctoral fellow at the University of Florida, continuing my research in pharmaceutical drug development.
In Florida, I became interested in the p-glycoprotein transporters and their key role in inhaled steroids drug development. I was fortunate to work under the able guidance of Prof. Gunther Hochhaus at the School of Pharmacy and later also contributed to several clinical studies led by Dr. Anthony Bavry, an interventional cardiologist who was my post-doctoral mentor.
In the last five years, though, my focus was on integrating this knowledge and applying it to real-world drug development strategies and regulatory interactions. I contributed to over a dozen early drug development programs for New Chemical Entity (NCE) as well as repurposed drugs. I had the opportunity to interact with the FDA in various divisions like anti-infectives, dermatology, cardiovascular and renal products, CNS and oncology. However, with all my translational drug development experience, I thought it was time to do something on my own and move on! Hence, ISHA Therapeutics was born.
PL: For readers that may not be so familiar with regulatory issues and drug development in the United States, could you summarize the main FDA-related regulatory processes and documents involved when starting a development program for a new drug candidate?
MJ: I will summarize the key pathways for regulatory approvals for drugs in the USA. Please note there are other products (e.g., biologicals, devices, etc.) which are not covered here.
Sponsors seeking to market their products (novel drugs, biologicals, generic drugs) in the USA need to obtain the approval from the US Food and Drug Administration (FDA).
There are three major regulatory drug approval pathways at the US-FDA.
In the 505 (b)(1) approval pathway a full new drug application (NDA) is submitted by the sponsor and the FDA reviews and approves it under section 505 (c) of the Food, Drug and Cosmetics (FD&C) act. This NDA consists of complete investigation reports of safety, effectiveness, and quality of the drug product under development. This pathway is used for the New Chemical Entities (NCE) and New Molecular Entities (NME). The sponsor needs to conduct the studies across non-clinical and clinical, ensuring the appropriate quality standards and submitting full reports of the studies performed across these key areas.
Another pathway is ANDA (Abbreviated New Drug Application), in which an application is submitted and approved under section 505(j) of the FD&C act for a drug product. This is also known as me-too products. This drug development pathway is less innovative compared to an NCE/NME, takes less time, and it has a lower cost. This is generally done when an RLD drug product goes off-patent and then it is available to be manufactured by anyone while demonstrating the bioequivalence to the innovator (RLD) product. In this pathway the sponsor can rely on the safety and efficacy data of the RLD.
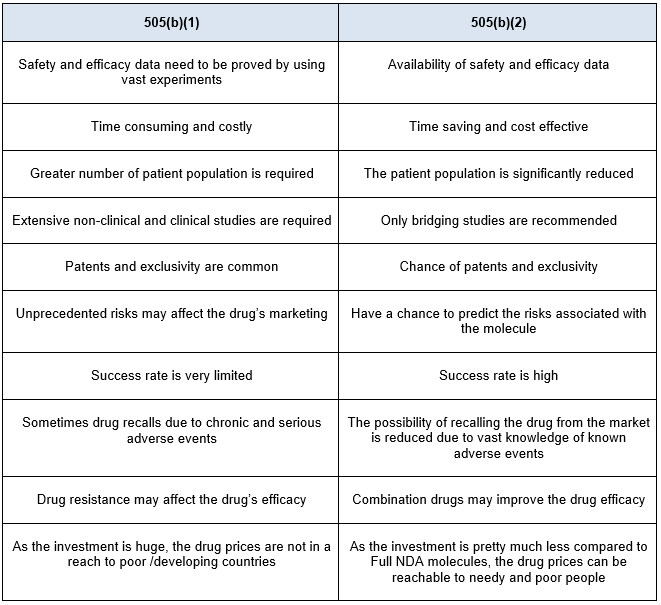
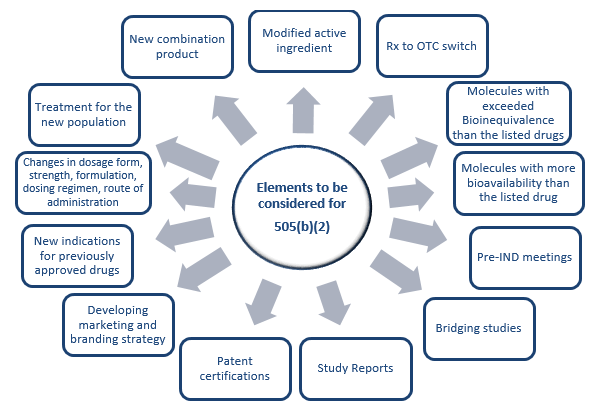
The other pathway is 505(b)(2). A 505(b)(2) application is an NDA submitted under section 505(b)(2) and approved under section 505(c) of the FD&C act, which contains full reports of investigations of safety and effectiveness, where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use. The 505(b)(2) regulation acts as a hybrid pathway between the full NDA and ANDA. Drug repurposing is the alternate pathway to identify drugs for new indications and new populations. There are several advantages of repurposing over developing a new chemical entity and these are listed in Table 1. Also, while repurposing one needs to consider several factors prior to undertaking such development. The details of such elements are provided in Figure 1 below.
Table 1. Advantages of 505(b)(2) pathway over 505(b)(1) pathway.
Figure 1. Key elements to be considered for repurposing.
PL: In the context of early phase drug development, what are some critical regulatory aspects that biotech companies should take very seriously when starting their drug development programs?
MJ: Drug development is a complex process and needs a detailed understanding of the disease, target population and the overall context of drug development across chemistry, non-clinical, and clinical development. It is very important to have right team members with key expertise and experience in drug development for both NCE as well as repurposed drug development programs. I will now elaborate more on the 505(b)(2) drug development strategies.
While repurposing the drugs under the 505(b)(2) regulatory pathways in the United States, one needs to be very critical. For example, we need a detailed understanding of the safety and efficacy of the approved molecule for its current indication as well as new changes that need to be implemented, like increasing solubility, changing dosage form, reducing the dose or dosing frequency, and improving patient compliance. You also need to assess how feasible it would be to run the clinical development program efficiently with minimum subjects being enrolled in the studies. We at ISHA prefer to reach out to the FDA, discuss the complete repurposing program with them, gain specific insights, advise, and then make a detailed analysis on what is feasible and what amount of time and money is needed to run that specific program. Once you have such a detailed analysis, the sponsor can make a go/no-go decision on the project.
It is also important to consider the current players in the market and the expected business share. One of the most important things is to consider how you are generating the intellectual property, which is not always obvious. This is a complex process and requires expertise across multiple domains. Fortunately, ISHA Therapeutics has all these experts available in house.
PL: What are some common regulatory difficulties or challenges that new (inexperienced) biotech companies may encounter when starting to develop a new compound? How can they avoid these complications?
MJ: New or inexperienced biotech companies may not have all the expertise and resources across the drug development spectrum at their early drug development journey. Hence, they at times struggle to get the right, scientifically enriched advice that can shape their drug development program. I can understand their challenges on the budget, administrative challenges, sustainability and less affordability to get all ducks lined up. Therefore, it is critical for early-stage companies to get the right guidance, develop the right strategies and ask the right questions on the feasibility of their ideas/scientific projects. It is good to kill the project as early as possible if it is not making scientific, product feasibility, and business sense. Hence, we provide our expertise and assist these companies to propel their drug discovery engines.
PL: Let’s talk about ISHA Therapeutics. How do you help biotech companies in their early stage drug development activities? Could you summarize your entire range of services?
MJ: We help early-stage companies to put their scientific program in the right direction with our key strategic inputs across non-clinical, clinical, and CMC domains of drug development in various key therapeutic areas. Details of our services are listed on our website. Briefly, we can help in providing advice to do integrated drug development, help conduct pre-IND meetings with CDER and CBER, two key divisions of US-FDA for drugs and biologicals respectively. We can also help the sponsors compile and file their INDs. Clinical development plan, feasibility analysis, expert opinion focusing on clinical and biopharmaceutics properties can be also provided.
PL: In the specific area of regulatory affairs, can you give a more detailed description of your capabilities?
MJ: We can help with integrated drug development strategies for non-clinical study design, its interpretations, and propose IND-enabling animal study rationale and design. We also work on clinical development plan, formulation development, target product profile, etc. We then help clients to integrate these strategies into pre-IND, IND compilation as submission with the US-FDA, kind of end-to end interactions. Details can be seen on our website.
PL: Let’s think of a new biotech start-up without experience interacting with the FDA. Could you explain the main meetings and interactions biotech companies have in the early stages of their drug development programs? Any advice to effectively deal with the FDA?
MJ: Very good question. In the early stage of drug development, a sponsor typically can initiate a formal interaction with the US-FDA via a formal pre-IND meeting (depending on the scope and objective there are three Types A, B and C meeting). This meeting is followed by filing an IND, which enables the sponsor to conduct human clinical studies (first in human study (FIH) for NCE and/or proof of concept Phase IIa/b clinical study for repurposed drugs depending on the objectives and scope) in the USA. Subsequently, the sponsors can meet with US-FDA through end of phase 2 meeting (EOP2) typically to discuss the plan for the registration Phase 3 clinical trial while discussing the Phase 2 results. There is also a pre-NDA meeting to discuss the submission and proposed label details. Our focus at this stage is on pre-IND, IND, EOP2.
PL: What about the Investigational New Drug (IND) document preparation? Could you give some essential recommendations to build a successful IND? According to your experience, what sections of the IND are of paramount importance?
MJ: Every section of each regulatory document is critical. There are set guidelines and a standard one needs to follow while compiling an IND document. Broadly, non-clinical, CMC, investigator’s brochure, and the proposed clinical development plan, including early-stage clinical study protocols, form the key component of an IND. Of course, there are several administrative, eCTD, and publication requirements to comply with the FDA standards.
PL: What kind of pharmaceutical compounds have you worked with? Do you have particular knowledge in terms of regulatory strategy for certain drug types?
MJ: We have worked for NCE as well as repurposed drugs across different therapeutic areas (e.g., CVS, CNS, Dermatology, Oncology, Infectious diseases e.g. COVID19, anti-fungal, etc.). Our team of senior advisors has impeccable experience in US-pharmaceutical industry, US-FDA and academia, which puts us into a strong position to provide inputs and strategy to any complex drug development program.
PL: Any final wise counsel you would like to share with biotech chief executive and scientific officers concerning regulatory strategy and drug development?
MJ: First, I would like to congratulate all the bold scientific executives, scientists who chose to embark on this unique journey to develop new drugs to fill in the gaps in the current unmet medical needs. To be closer to success in drug development we must be emotionally detached from our innovation, and at the same time extremely unbiased with the scientific feasibility of the innovation you are involved in. If you put the right strategies at the right stage of drug development then success is going to be yours. Engage with the regulatory agencies as early as possible to get the right guidance, advice, and direction. Then you are sure on the specific path you are moving, where you can then confidently invest your time, energy, and resources to be able to move to the next stage of drug development.
PL: Manoj, thanks so much for all your insights. I am sure this information will be helpful for biotech companies navigating the early stages of drug development.
MJ: Thank you! It was my pleasure.
About ISHA Therapeutics
ISHA Therapeutics is a unique organization with its primary location based in New Jersey, exclusively focused on supporting clinical and translational development programs spanning from very early on to late stage developments. We provide consulting services to our esteemed clients who aspire to develop safe and efficacious new drugs, devices, and biologicals. We also have our in-house innovative drug development pipeline.
For further information about regulatory services and drug development in the United States, please contact us at: info@sofpromed.com