Biotech and pharma companies planning clinical trials in Europe will have to submit a clinical trial application (CTA) to the national regulatory authorities of the countries in which the clinical study is to be conducted.
What are the documents needed for regulatory submissions in the EU?
The EU Regulation 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use provides complete guidance on the documentation to be included in the clinical trial submission package to be sent to regulatory authorities in EU countries.
This article seeks to provide to-the-point practical help to clinical trial sponsors, so that they can have a clear view of the key documents they must prepare.
Understanding the General Structure of the Clinical Trial Application: Parts I and II
The clinical trial application documentation in the EU is structured in two parts: Part I and Part II.
Part I contains the documents to be submitted to the regulatory authority (some of them will be reviewed by the Ethics Committee as well), while Part II only deals with documentation to be sent to the Ethics Committee.
A clinical trial application in the EU must be evaluated by both, the regulatory authority and the Ethics Committee. In this article we are exclusively focused on the documents to be submitted to the regulatory authority alone.
The XML File for a European Clinical Trial Application
Another initial aspect to be understood is the generation of the XML file for the clinical trial application (CTA), which contains several forms with the clinical trial information. The XML file can be created and completed online via the EudraCT web site.
This is a brief summary of the sections included in the XML CTA:
A. Trial Identification
B. Sponsor Identification
C. Applicant Identification
D. IMP Identification
D. 8 Placebo Information
D. 9 Site(s) where the qualified person certifies batch release
E. General Information on the Trial
F. Population of Trial Subjects
G. Clinical Trial Sites/Investigators in the Member State
H. Competent Authority/Ethics Committee Information
The clinical trial XML file is the building block of the trial application and it will be used to carry out the submission in every participating country.
The regulatory authorities of each EU member state usually have a web-based system or portal used to perform clinical trial application submissions, and these electronic systems require the upload of the completed XML file.
List of Key Documents Required for the Initial Trial Application to the National Regulatory Authority
In short, these are the key documents you will need to submit the initial trial application to the regulatory authority:
- Clinical trial application cover letter
- Clinical trial application form
- Study protocol
- Investigator’s Brochure (IB) and/or Summary of Product Characteristics (SmPC)
- GMP-related documents (manufacturer/importer authorizations, Qualified Person Declaration)
- Investigational Medicinal Product Dossier (IMPD)
- Drug labels
- Evaluation fees
There might be some study-specific additional documentation to be prepared, but the above list provides an essential checklist for the most important documents.
Let’s proceed to discuss each of these basic pieces of information.
1. Clinical Trial Application Cover Letter
The trial application cover letter is normally completed and generated through the web-based portal of each regulatory authority. In addition, some data expected in the cover letter may already be contained in the EU application form. Then, the information to be included in the cover letter (according to EU guidelines) will be mostly produced electronically.
The cover letter shall specify the EU trial number and the universal trial number and shall draw attention to any particular characteristics of the clinical trial acording to EU instructions (Regulation 536/2014), the cover letter shall include the following aspects:
(a) specific features of the clinical trial population, such as subjects not able to give informed consent, minors and pregnant or breastfeeding women;
(b) whether the clinical trial involves the first administration of a new active substance to humans;
(c) whether scientific advice relating to the clinical trial or the investigational medicinal product has been given by the Agency, a Member State or a third country;
(d) whether the clinical trial is part or is intended to be part of a Paediatric Investigation Plan (PIP) as referred to in Title II, Chapter 3, of Regulation (EC) No 1901/2006 (if the Agency has already issued a decision on the PIP, the cover letter contains the link to the decision of the Agency on its website);
(e) whether investigational medicinal products or auxiliary medicinal products are a narcotic, psychotropic or radiopharmaceutical;
(f) whether the investigational medicinal products consist of or contain a genetically-modified organism or organisms;
(g) whether the sponsor has obtained an orphan designation for the investigational medicinal product for an orphan condition;
(h) a comprehensive list, including the regulatory status, of all investigational medicinal products and a list of all auxiliary medicinal products; and
(i) a list of medical devices which are to be investigated in the clinical trial but which are not part of the investigational medicinal product or products, together with a statement as to whether the medical devices are CE-marked for the intended use.
2. Clinical Trial Application Form
The clinical trial application form is the EU XML file in PDF format. The web portals used to build the CTA and perform the trial submission to competent authorities allow the conversion of the XML contents into the PDF application document.
3. Study Protocol
Based on Regulation (EU) No 536/2014, the protocol shall describe objective, design, methodology, statistical considerations, purpose and organization of the clinical trial.
The protocol shall be identified by:
(a) the title of the clinical trial;
(b) the EU trial number;
(c) the sponsor’s protocol code number specific for all versions of it (if relevant);
(d) the date and number of the version, to be updated when it is amended;
(e) a short title or name assigned to the protocol; and
(f) the name and address of the sponsor, as well as the name and function of the representative or representatives of the sponsor authorized to sign the protocol or any substantial modification to the protocol.
The protocol shall at least include the following information [please note this is an adapted, summarized version of the detailed list included in Regulation (EU) No 536/2014. Refer to the mentioned regulation for the full version of this list]:
(a) a statement that the clinical trial is to be conducted in compliance with the protocol, with EU regulations and with the principles of good clinical practice;
(b) a comprehensive list of all investigational medicinal products and of all auxiliary medicinal products;
(c) a summary of findings from non-clinical studies that potentially have clinical significance and from other clinical trials that are relevant to the clinical trial;
(d) a summary of the known and potential risks and benefits including an evaluation of the anticipated benefits and risks;
(e) where patients were involved in the design of the clinical trial, a description of their involvement;
(f) a description of, and justification for, the dosage, the dosage regime, the route and mode of administration, and the treatment period for all investigational medicinal products and auxiliary medicinal products;
(g) a statement of whether the investigational medicinal products and auxiliary medicinal products used in the clinical trial are authorized;
(h) a description of the groups and subgroups of the subjects participating in the clinical trial;
(i) references to literature and data that are relevant to the clinical trial, and that provide background for the clinical trial;
(j) a discussion of the relevance of the clinical trial;
(k) a description of the type of clinical trial to be conducted and a discussion of the trial design (including a schematic diagram of trial design, procedures and stages, if relevant);
(l) a specification of the primary end-points and the secondary end-points, if any, to be measured during the clinical trial;
(m) a description of the measures taken to minimize bias, including, if applicable, randomization and blinding;
(n) a description of the expected duration of subject participation and a description of the sequence and duration of all clinical trial periods, including follow-up, if relevant;
(o) a clear and unambiguous definition of the end of the clinical trial;
(p) a description of the criteria for discontinuing parts of the clinical trial or the entire clinical trial;
(q) arrangements for the maintenance of clinical trial treatment randomization codes and procedures for breaking codes, if relevant;
(r) a description of procedures for the identification of data to be recorded directly on the Case Report Forms considered as source data;
(s) a description of the arrangements to comply with the applicable rules for the collection, storage and future use of biological samples from clinical trial subjects, where applicable, unless contained in a separate document;
(t) a description of the arrangements for tracing, storing, destroying and returning the investigational medicinal product and unauthorized auxiliary medicinal product;
(u) a description of the statistical methods to be employed, including, if relevant:
— timing of any planned interim analysis and the number of subjects planned to be enrolled;
— reasons for choice of sample size;
— calculations of the power of the clinical trial and clinical relevance;
— the level of significance to be used;
— criteria for the termination of the clinical trial;
— procedures for accounting for missing, unused, and spurious data and for reporting any deviation from the original statistical plan; and
— the selection of subjects to be included in the analyses;
(v) a description of the subject inclusion and exclusion criteria, including criteria for withdrawing individual subjects from treatment or from the clinical trial;
(w) a description of procedures relating to the withdrawal of subjects from treatment or from the clinical trial including procedures for the collection of data regarding withdrawn subjects, procedures for replacement of subjects and the follow-up of subjects that have withdrawn from treatment or from the clinical trial;
(x) a justification for including subjects who are incapable of giving informed consent or other special populations, such as minors;
(y) a justification for the gender and age allocation of subjects and, if a specific gender or age group is excluded from or underrepresented in the clinical trials, an explanation of the reasons and justification for these exclusion criteria;
(z) a detailed description of the recruitment and informed consent procedure, especially when subjects are incapable of giving informed consent;
(aa) a description of the treatments, including medicinal products, which are permitted or not permitted, before or during the clinical trial;
(ab) a description of the accountability procedures for the supply and administration of medicinal products to subjects including the maintenance of blinding, if applicable;
(ac) a description of procedures for monitoring subject compliance, if applicable;
(ad) a description of arrangements for monitoring the conduct of the clinical trial;
(ae) a description of the arrangements for taking care of the subjects after their participation in the clinical trial has ended, where such additional care is necessary because of the subjects’ participation in the clinical trial and where it differs from that normally expected for the medical condition in question;
(af) a specification of the efficacy and safety parameters as well as the methods and timing for assessing, recording, and analysing these parameters;
(ag) a description of ethical considerations relating to the clinical trial if those have not been described elsewhere;
(ah) a statement from the sponsor (either in the protocol or in a separate document) confirming that the investigators and institutions involved in the clinical trial are to permit clinical trial-related monitoring, audits and regulatory inspections, including provision of direct access to source data and documents;
(ai) a description of the publication policy;
(aj) duly substantiated reasons for the submission of the summary of the results of the clinical trials after more than one year;
(ak) a description of the arrangements to comply with the applicable rules on the protection of personal data;
(al) a description of measures that will be implemented to ensure confidentiality of records and personal data of subjects;
(am) a description of measures that will be implemented in case of data security breach.
The protocol shall also describe the procedures for:
(a) eliciting and recording adverse events by the investigator, and the reporting of relevant adverse events by the investigator to the sponsor;
(b) reporting by the investigator to the sponsor of those serious adverse events which have been identified in the protocol as not requiring immediate reporting;
(c) reporting of suspected unexpected serious adverse reactions by the sponsor to the Eudravigilance database; and
(d) follow-up of subjects after adverse reactions including the type and duration of follow-up.
4. Investigator’s Brochure (IB) and/or Summary of Product Characteristics (SmPC)
In the context of a clinical trial, regulatory authorities require the submission of the investigator’s brochure (IB) and/or the Summary of Product Characteristics (SmPC) of the drugs under investigation.
According to Annex I of Regulation (EU) No 536/2014 (Section E; 26, 27, 28):
“The purpose of the IB is to provide the investigators and others involved in the clinical trial with information to facilitate their understanding of the rationale for, and their compliance with, key features of the protocol, such as the dose, dose frequency/interval, methods of administration, and safety monitoring procedures.
The information in the IB shall be presented in a concise, simple, objective, balanced and non-promotional form that enables a clinician or investigator to understand it and make an unbiased risk-benefit assessment of the appropriateness of the proposed clinical trial. It shall be prepared from all available information and evidence that supports the rationale for the proposed clinical trial and the safe use of the investigational medicinal product in the clinical trial and be presented in the form of summaries.
If the investigational medicinal product is authorised, and is used in accordance with the terms of the marketing authorisation, the approved summary of product characteristics (SmPC) shall be the IB. If the conditions of use in the clinical trial differ from those authorised, the SmPC shall be supplemented with a summary of relevant non-clinical and clinical data that support the use of the investigational medicinal product in the clinical trial. Where the investigational medicinal product is identified in the protocol only by its active substance, the sponsor shall select one SmPC as equivalent to the IB for all medicinal products that contain that active substance and are used at any clinical trial site.”
5. GMP-related Documents (Manufacturer/Importer Authorizations, Qualified Person Declaration)
Regarding the documents related to GMP compliance of the drug product to be used in the clinical trial, no documentation is to be submitted to the regulatory authority if the drug is authorized in the EU and is not modified, whether or not it is manufactured in Europe.
If the investigational medicinal product (IMP) is not authorized in the EU, and is not manufactured in Europe, the following documents will be required:
(a)A copy of the importer/manufacturer authorization: This is an authorization granted by EU national regulatory authorities to the local companies (e.g. depots, CDMOs) in charge of drug product import, manufacturing, testing, and handling.
(b)QP Declaration: A certification by the qualified person (QP) in the EU that the manufacturing complies with GMP at least equivalent to the GMP in Europe. For more information about the QP Declaration, please read this article.
In all other cases, a copy of the manufacturer authorization shall be submitted.
6. Investigational Medicinal Product Dossier (IMPD)
The IMPD is a very important document that provides data on the quality of IMPs, including drug substance and drug product manufacturing, testing, analysis, and control aspects, as well as data related to non-clinical and clinical studies.
Quality data shall be submitted according to the Guideline on the requirements for the chemical and pharmaceutical quality documentation concerning investigational medicinal products in clinical trials.
The IMPD shall also contain summaries of non-clinical pharmacology and toxicology data for any investigational medicinal product used in the clinical trial. It shall contain a reference list of studies conducted and appropriate literature references.
Non-clinical pharmacology and toxicology data shall be submitted in a logical structure, such as that of Module 4 of the ICH Common Technical Document format.
Data from previous clinical trials and human experience shall be submitted according to Module 5 of the ICH Common Technical Document format.
The IMPD will also include an overall risk and benefit assessment in the form of a summary that critically evaluates the non-clinical and clinical data in relation to the potential risks and benefits of the investigational medicinal product in the proposed clinical trial unless this information is already provided in the protocol.
7. Drug Labels
Study drug label designs must be submitted to regulatory authorities when sending the clinical trial application.
Following the general rules of Annex VI (EU Regulation 536/2014) for unauthorized IMPs (section A.1), this information must appear on the immediate and the outer packaging of the drug:
(a) name, address and telephone number of the main contact for information on the product, clinical trial and emergency unblinding; this may be the sponsor, contract research organisation or investigator (for the purpose of this Annex this is referred to as the ‘main contact’);
(b) the name of the substance and its strength or potency, and in the case of blind clinical trials the name of the substance is to appear with the name of the comparator or placebo on the packaging of both the unauthorised investigational medicinal product and the comparator or placebo;
(c) pharmaceutical form, route of administration, quantity of dosage units;
(d) the batch or code number identifying the contents and packaging operation;
(e) a clinical trial reference code allowing identification of the trial, site, investigator and sponsor if not given elsewhere;
(f) the subject identification number and/or the treatment number and, where relevant, the visit number;
(g) the name of the investigator (if not included in (a) or (e));
(h) directions for use (reference may be made to a leaflet or other explanatory document intended for the subject or person administering the product);
(i) ‘For clinical trial use only’ or similar wording;
(j) the storage conditions;
(k) period of use (expiry date or re-test date as applicable), in month and year format and in a manner that avoids any ambiguity; and
(l) ‘Keep out of reach of children’, except when the product is for use in trials where the product is not taken home by subjects.
8. Evaluation Fees
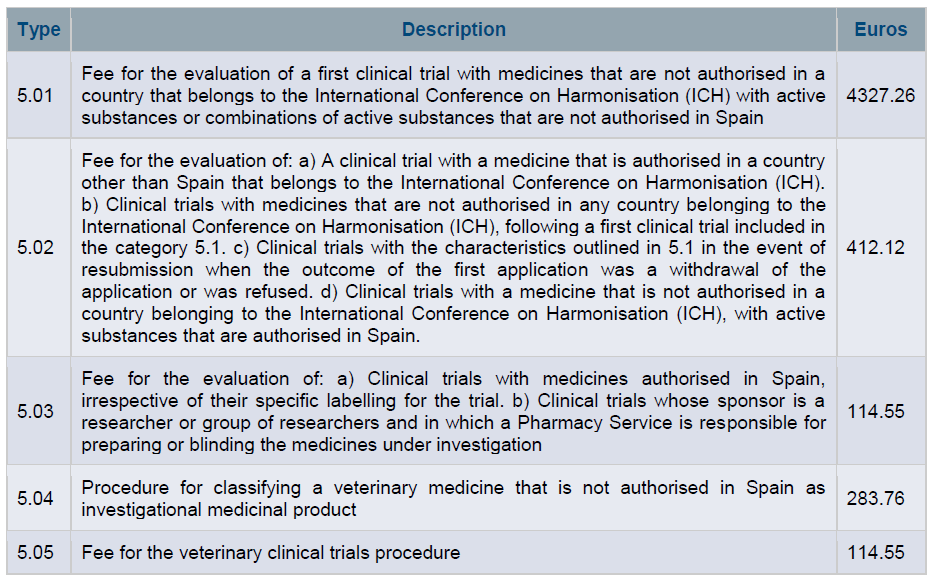
Finally, regulatory authorities will normally charge fees for the efforts made to evaluate a clinical trial application. Different prices may apply depending on the characteristics of the clinical study. For instance, you can check the fees of the Spanish regulatory authority (Spanish Agency of Medicines and Medical Devices, AEMPS) in the image below.
Regulatory authorities normally allow fee payments via credit card. The fee payment receipts are usually included in the submission package.
Conclusion
The key documents for a clinical trial application to regulatory authorities in the EU are: the cover letter, the application form, the study protocol, the IB/SmPC, the manufacturer/importer authorizations, the Qualified Person Declaration, the IMPD, the drug labels, and the evaluation fees (payment receipts).
Clinical trial applications to regulatory authorities in Europe may have particular local challenges, so counting on the support of local regulatory specialists is highly recommended.
Please contact us at info@sofpromed.com if you need help to submit a clinical trial application in Europe.