Contact us at info@sofpromed.com if you need an EDC system for your clinical trial.
An electronic data capture (EDC) system —also referred to as electronic case report form (eCRF)— is a very important software tool in clinical trials.
Clinical research relies on accurate data and EDC solutions are used to collect, clean, and analyze the data produced in clinical studies.
In this article you will learn about technical and practical aspects related to EDC systems, including costs for their acquisition and use in clinical trials.
What is an electronic data capture (EDC) system in clinical trials?
An electronic data capture (EDC) system —also known as electronic case report form (eCRF)— is a web-based software application used to collect, clean, transfer, and process data in clinical trials.
Is an electronic data capture (EDC) system the same as an eCRF?
Even though the concept of “EDC” has wider implications, in the context of clinical trials, an EDC system is considered to be the same as an eCRF.
Is it mandatory to use an EDC software in clinical trials?
There is no legal or regulatory obligation to use an EDC software in a clinical trial.
Moreover, EDC systems are relatively new technologies and there was a time when clinical trials were conducted without EDCs.
For example, clinical data used to be collected using paper-based forms, something still done today (although less frequently).
In any case, clinical data has to be collected by some means and nowadays EDC solutions are the best option.
What are the advantages of using an EDC system in clinical trials?
Using an EDC system certainly provides a number of advantages when conducting a clinical trial. Let’s mention some of them.
Firstly, utilizing an EDC solution streamlines data collection when compared to paper-based data collection. Clinical sites can access the EDC system from any computer and enter data quickly and easily, making it immediately available to data reviewers. This avoids the hassle of writing and shipping paper forms.
Secondly, EDC tools accelerate the data cleaning process. Data managers can easily review the data entered in the system and issue queries to sites to solve discrepancies.
Thirdly, an EDC platform guarantees solid data authenticity, integrity, and security, since it integrates advanced mechanisms to manage access controls and data traceability.
What kind of data is collected in EDC systems?
EDC systems collect different types of data depending on the therapeutic area of the clinical trial.
For instance, in oncology studies the following data forms are typically used: demographics, medical history, vital signs, ECOG performance status, electrocardiogram data, biochemistry, hematology, coagulation tests, urinalysis, pregnancy tests, adverse events, concomitant medications, tumor assessments, treatment data, survival follow-up, and death information, among others.
How is data collected in an EDC software?
An EDC software usually comes in the form of a web-based application without the need of installing anything else in the computer.
This means that users only have to access the EDC system via a web browser using their usernames and passwords.
Once logged in the application, users will type the clinical data in the fields contained in the various data forms.
Therefore, data entry in an EDC tool is done remotely by local site users, who extract the patient data from the site’s source documents (medical records).
How is data cleaned in an EDC system?
Once the clinical data has been entered in the EDC web-based platform by the hospital’s personnel, it is time to refine this information by detecting and solving discrepancies.
The process of identifying and correcting missing or inconsistent information is known as “data cleaning”.
Data cleaning in clinical trials is typically performed by data managers belonging to the vendor in charge of data management (e.g. CRO staff).
These data managers will periodically review the EDC information and will issue queries to sites, asking them to clarify and/or correct data discrepancies.
This process is repeated until all the data is complete, clear, and consistent, so that it can be exported and analyzed.
How is data exported for analysis in an EDC software?
After the clinical data entered in an EDC system has been cleaned, the next step is to export this information for further analysis.
EDC applications normally integrate data export mechanisms to generate dataset files that can be in turn used in statistical software tools (e.g. SAS, SPSS).
The clinical information contained in EDC platforms is structured in datasets, and the details of the export processes are documented beforehand, in the early stages of the clinical trial.
How and how often will the data be exported? In what format? These questions are normally addressed in the Data Management Plan (DMP).
What are some of the advanced features that EDC software products have?
EDC software products offer more and more sophisticated and powerful features every day.
In the clinical research sector you can find EDC solutions with different levels of sophistication and functionality.
Evidently, the biggest EDC vendors in the industry tend to have the more powerful products as they have been improving their solutions for years.
EDC systems include advanced features to perform different tasks. For instance, some advanced functionalities include: automatic query mechanisms, automated coding of medical terms, generation of customized reports, and powerful modules for data cleaning and source data verification.
An example of a very interesting advanced feature in an EDC is the Anju Software’s AutoEncoder tool.
When conducting clinical trials or monitoring safety for approved products, the regulatory agencies require that adverse events be coded against the MedDRA dictionary, and that medications be coded against the WHODrug dictionary.
The source terms are usually entered in free text, and the coding process is performed by specialist medical professionals, who must ensure consistent and accurate decisions based on the content of the source term, any relevant related data, and prior decisions for the trial.
The Anju AutoEncoder provides a modern and sophisticated tool for performing these coding activities. Out of the box it is fully integrated with Anju’s TrialMaster® product for Electronic Data Capture (EDC), but its open interfaces allow for integration with any third-party EDC or safety reporting system.
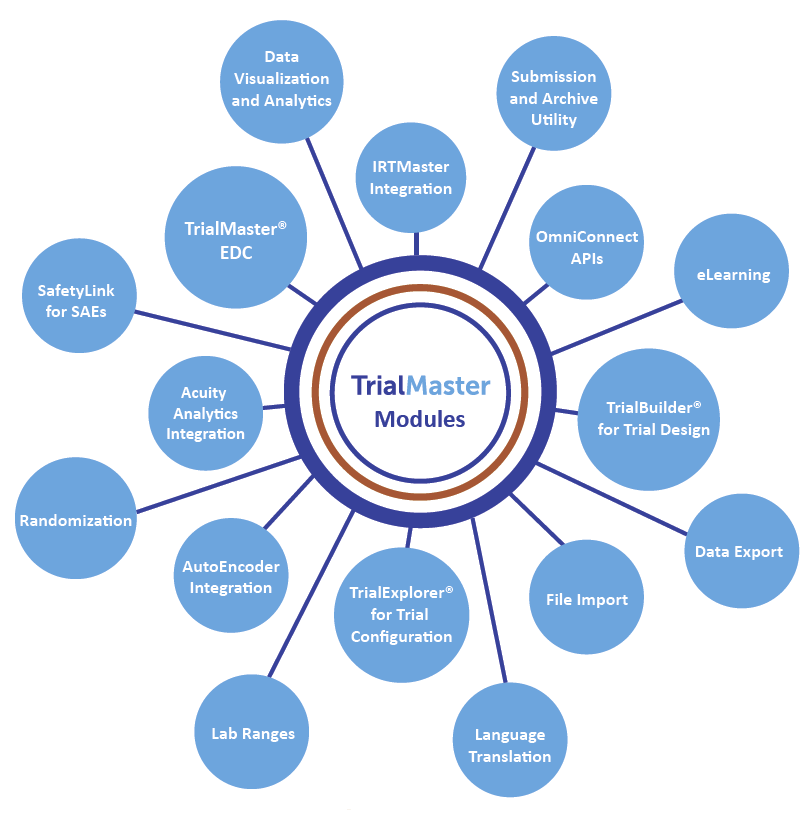
Additional features of the TrialMaster software can be seen in the figure below.
TrialMaster Modules
How are EDC systems designed and implemented?
EDC systems require a specialized and strict design and implementation process for each clinical trial.
A number of plans have to be written to document the design and implementation procedures, including a Data Management Plan (DMP) and an annotated CRF (aCRF) document.
A DMP is a document that helps data managers and statistical programmers to understand the type of data that will be produced in a clinical trial, and how this data will be collected, stored, cleaned, exported, and analyzed.
An aCRF is a document that maps the clinical data collection fields used to capture patient data to the corresponding variables contained within the Study Data Tabulation Model (SDTM) datasets.
Essentially, the EDC system design and implementation process consists of developing the system specifications and then actually building the software system itself (implementing, testing, and validating the data forms and fields) according to such requirements.
These activities are performed by the data managers of the company in charge of building the EDC system (e.g. CRO staff).
What regulatory requirements should EDC solutions meet?
The regulatory requirements that EDC systems must meet have been established in the United States through the part 11 of Title 21 of the Code of Federal Regulations; Electronic Records; Electronic Signatures (21 CFR Part 11).
To achieve regulatory compliance of EDC systems, the following technical controls must be in place:
- Technical controls: Measures that ensure the quality, accuracy, and integrity of data stored in the electronic systems.
- User authorization controls: Security measures to identify the person who accesses the application and submits the data, to prevent unauthorized access to the system.
- Audit trail controls: Measures to ensure that the system keeps a record about sources from which data originates, who made changes, when, and what information was changed.
- Attributability controls: Measures to ensure that data will be retrievable in such a way that all information regarding each subject in a study is attributable to that subject.
- Data validation controls: Checks performed by the computerized systems to ensure the validity and quality of clinical information.
Finally, system integrity measures must be implemented to guarantee the integrity of the system and protect against data loss (e.g. periodic backups).
What are some of the main security and data quality features EDC systems must have?
EDC systems should at least have the following mechanisms to ensure data security and traceability: an internal clock (date and time), an access control module, and an activity registry.
The internal clock system should consistently show the current date and time, so that all the activity taking place in the system is recorded with the right time.
Secondly, an access control module should be available to track each user’s access date and time, the user identity, the IP address used, and the type of access (e.g. login, logout, time out, login error).
Additionally, an activity registry must be in place to track the activity through the EDC pages. For each action in the system, data such as Subject ID, username, variable name, inserted value, form name, as well as the date and time of the action should be recorded.
What are the best EDC systems?
Some of the best and most widely used EDC systems worldwide are Rave from Medidata, Inform from Oracle, and TrialMaster from Anju Software.
How big is the EDC system market in the United States?
According to this Electronic Data Capture Systems Market Size, Share & Trends Analysis Report:
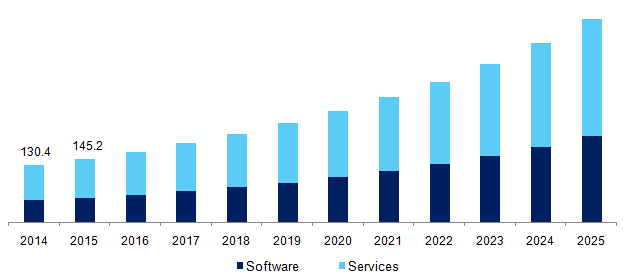
“The global Electronic Data Capture systems market size was valued at USD 349.8 million in 2016. The increasing complexity in the management of clinical information generated before, during, and after the trial is expected to propel the demand for EDC systems over the forecast period. The statistics published by the Center for Study of Drug Development states that more than 80% of clinical trials failed as they are unable to meet regulatory requirements, which resulted in delayed drug commercialization. This resulted in a loss of revenue for manufacturers. EDC systems help curb this problem by capturing and managing clinical information simply.”
U.S. EDC systems market, by component, 2014 – 2025 (USD Million)
How much does an EDC software cost?
The pricing of EDC software tools can vary depending on the level of quality and the number of advanced features each system has.
The reader is encouraged to check the article “How Much Does an Electronic Case Report Form (eCRF) Cost?” to learn about EDC system costs.
Please contact us at info@sofpromed.com if you need an EDC system quotation.